Design of a phase III, double-blind, randomised, placebo-controlled trial of BI 1015550 in patients with idiopathic pulmonary fibrosis (FIBRONEER-IPF)
- PMID: 37597969
- PMCID: PMC10441083
- DOI: 10.1136/bmjresp-2022-001563
Design of a phase III, double-blind, randomised, placebo-controlled trial of BI 1015550 in patients with idiopathic pulmonary fibrosis (FIBRONEER-IPF)
Abstract
IntroductionThere is an unmet need for new treatments for idiopathic pulmonary fibrosis (IPF). The oral preferential phosphodiesterase 4B inhibitor, BI 1015550, prevented a decline in forced vital capacity (FVC) in a phase II study in patients with IPF. This study design describes the subsequent pivotal phase III study of BI 1015550 in patients with IPF (FIBRONEER-IPF).
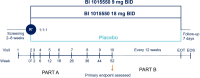
Methods and analysis: In this placebo-controlled, double-blind, phase III trial, patients are being randomised in a 1:1:1 ratio to receive 9 mg or 18 mg of BI 1015550 or placebo two times per day over at least 52 weeks, stratified by use of background antifibrotics (nintedanib/pirfenidone vs neither). The primary endpoint is the absolute change in FVC at week 52. The key secondary endpoint is a composite of time to first acute IPF exacerbation, hospitalisation due to respiratory cause or death over the duration of the trial.
Ethics and dissemination: The trial is being carried out in compliance with the ethical principles of the Declaration of Helsinki, in accordance with the International Council on Harmonisation Guideline for Good Clinical Practice and other local ethics committees. The results of the study will be disseminated at scientific congresses and in peer-reviewed publications.
Trial registration number: NCT05321069.
Keywords: Lung Transplantation.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: LR has received research grants from Boehringer Ingelheim and the Italian Medicine Agency; has been an advisory board member for Roche, Boehringer Ingelheim, FibroGen, Biogen and Promedior; has been involved in consulting activity for Biogen, Celgene, Nitto, Pliant Therapeutics, Toray, BMS, RespiVant, Zambon and CSL Behring; has received payment for lectures from Boehringer Ingelheim, Zambon and Cipla; has received support for attending meetings from Boehringer Ingelheim and Roche; and been a steering committee member for Boehringer Ingelheim and Roche. AA has received research grants and speaker fees from Boehringer Ingelheim and Taiho Pharm. Co.; and has been an advisory committee member for Boehringer Ingelheim, Taiho Pharm. Co., Toray Medical Co. and Kyorin Pharm. Co. VC has received unrestricted grants from Boehringer Ingelheim; consulting fees from AstraZeneca, Boehringer Ingelheim, Celgene/BMS, CSL Behring, Ferrer, Galapagos, Pliant Therapeutics, PureTech, RedX, Roche, Sanofi and Shionogi; lecture fees from Boehringer Ingelheim and Roche; support for attending meetings from Boehringer Ingelheim and F. Hoffmann-La Roche; has participated in data and safety monitoring boards for Galapagos, Galecto and Roche; and has been on an adjudication committee for FibroGen. MK is an advisor or review panel member for Boehringer Ingelheim, Galapagos and Roche; and has received consultancy fees, grants and speaker fees from Boehringer Ingelheim and Roche. TMM has received consulting fees from Boehringer Ingelheim, Roche/Genentech, AstraZeneca, Bayer, Blade Therapeutics, Bristol Myers Squibb, Galapagos, Galecto, GSK, IQVIA, Pliant Therapeutics, Respivant, Theravance Biopharma and Veracyte. He has also received speaker fees from Boehringer Ingelheim and Roche/Genentech. FJM has served on a steering committee, advisory board, data safety monitoring board or adjudication committee for Afferent/Merck, Bayer, Biogen, Boehringer Ingelheim, DevPro, Nitto, Novartis, Respivant, Roche and Veracyte; and has received consulting fees or payment for presentations from AbbVie, Boehringer Ingelheim, Bristol Myers Squibb, Bridge Biotherapeutics, CSL Behring, DevPro, IQVIA, Lung Therapeutics, Roche/Genentech, Sanofi, Shionogi, twoXAR, United Therapeutics and Veracyte. JMO has received consultancy fees and grants from Boehringer Ingelheim and consultancy fees from Roche/Genentech and Lupin Pharmaceuticals. MG, YL, SS and DFZ are employees of Boehringer Ingelheim. CV has received personal fees from Boehringer Ingelheim, F. Hoffmann-La Roche and Bristol Myers Squibb; and support for attending meetings from Boehringer Ingelheim and F. Hoffmann-La Roche. MSW has received grants from AstraZeneca-Daiichi, Boehringer Ingelheim, F. Hoffmann-La Roche, The Netherlands Organisation for Health Research and Development, The Dutch Lung Foundation and The Dutch Pulmonary Society; consulting fees from Boehringer Ingelheim, Galapagos, Bristol Myers Squibb, Galecto, Respivant and NeRRe Therapeutics, Horizon Therapeutics, PureTech Health, Kinevant Sciences, Molecure, CSL Behring, Thyron and Vicore; speaker fees from Boehringer Ingelheim, Galapogas, F. Hoffmann-La Roche, Novartis and CSL Behring; support for attending meetings from Boehringer Ingelheim, Galapagos and F. Hoffmann-La Roche; has participated in advisory boards for Savara, Galapagos and Dutch lung fibrosis and sarcoidosis patient associations (unpaid); and has held leadership roles as Chair of the Idiopathic Interstitial Pneumonia group of the European Respiratory Society, Member of the board of the Netherlands Respiratory Society, Member of the scientific advisory board of the European Idiopathic Pulmonary Fibrosis and Related Disorders Federation and Chair of the educational committee of the European Reference Network for Rare Lung Diseases. All grants and fees were paid to her institution.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
