CD98hc is a target for brain delivery of biotherapeutics
- PMID: 37598178
- PMCID: PMC10439950
- DOI: 10.1038/s41467-023-40681-4
CD98hc is a target for brain delivery of biotherapeutics
Erratum in
-
Author Correction: CD98hc is a target for brain delivery of biotherapeutics.Nat Commun. 2023 Sep 7;14(1):5516. doi: 10.1038/s41467-023-41355-x. Nat Commun. 2023. PMID: 37679403 Free PMC article. No abstract available.
Abstract
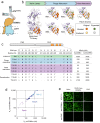
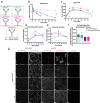
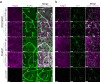
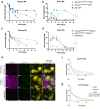
Brain exposure of systemically administered biotherapeutics is highly restricted by the blood-brain barrier (BBB). Here, we report the engineering and characterization of a BBB transport vehicle targeting the CD98 heavy chain (CD98hc or SLC3A2) of heterodimeric amino acid transporters (TVCD98hc). The pharmacokinetic and biodistribution properties of a CD98hc antibody transport vehicle (ATVCD98hc) are assessed in humanized CD98hc knock-in mice and cynomolgus monkeys. Compared to most existing BBB platforms targeting the transferrin receptor, peripherally administered ATVCD98hc demonstrates differentiated brain delivery with markedly slower and more prolonged kinetic properties. Specific biodistribution profiles within the brain parenchyma can be modulated by introducing Fc mutations on ATVCD98hc that impact FcγR engagement, changing the valency of CD98hc binding, and by altering the extent of target engagement with Fabs. Our study establishes TVCD98hc as a modular brain delivery platform with favorable kinetic, biodistribution, and safety properties distinct from previously reported BBB platforms.
© 2023. Springer Nature Limited.
Conflict of interest statement
K.S.C., R.C.W., A.M., D.C., K.J.L., H.L.T., J.C., D.J.K., Y.R.C., D.B.S., R.K.T., M.T., K.X., A.Y., Y.Z., P.A., L.A., G.M.C., T.K.E., A.G., D.H., D.J., K.N.K., D.L., A.W.S.L., K.W.L., N.P.D.L., I.B., J.M., H.N.N., E.I.L., M.E.P., E.R., P.S., M.E.K.C., M.S.D., J.D., K.G., J.W.L., C.S.M., R.M., H.S., R.G.T., R.J.W., Y.J.Y.Z., M.S.K. are paid employees of Denali Therapeutics Inc. Denali has filed patent application no. PCT/US2022/053220 related to the subject matter of this paper, which includes the discovery and application of the CD98hc TVs. K.S.C., R.C.W., H.L.T., P.A., G.M.C., M.S.D., Y.J.Y.Z. and M.S.K. are inventors of this patent application. There are no other competing interests.
Figures



References
-
- Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ. Structure and function of the blood-brain barrier. Neurobiol. Dis. 2010;37:13–25. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

