Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice
- PMID: 37598463
- PMCID: PMC10462885
- DOI: 10.1016/j.redox.2023.102852
Remote ischemic conditioning attenuates oxidative stress and inflammation via the Nrf2/HO-1 pathway in MCAO mice
Abstract
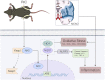
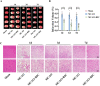
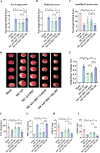
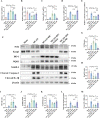
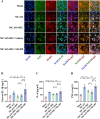
The protective effects of remote ischemic conditioning (RIC) on acute ischemic stroke have been reported. However, the protective mechanisms of RIC have not been fully elucidated. This study aimed to investigate whether RIC could reduce oxidative stress and inflammatory responses in middle cerebral artery occlusion (MCAO)-reperfusion mice via the nuclear factor-E2-related factor 2 (Nrf2)/heme oxygenase-1 (HO-1) pathway. C57BL/6 mice were subjected to MCAO and underwent RIC twice daily at 1, 3, and 7 days after MCAO. ML385 was used to specifically inhibit Nrf2 in MCAO mice. Neurological deficit scores, infarct volume, and hematoxylin-eosin (HE) staining were assessed. Oxidative stress levels were assessed based on total antioxidant capacity (TAC), malonaldehyde (MDA), superoxide dismutase (SOD), and glutathione/glutathione disulfide (GSH/GSSG). mRNA levels were detected using real-time polymerase chain reaction (PCR), and protein levels were detected using western blotting and enzyme-linked immunosorbent assay (ELISA). Protein localization was investigated using immunofluorescence staining. RIC significantly reduced infarct volume and improved neurological function and histological changes after MCAO. RIC significantly increased TAC, SOD, and GSH/GSSG levels and decreased MDA levels. RIC significantly increased Nrf2 and HO-1 mRNA levels and decreased Keap1, NLRP3, and Cleaved Caspase-1 mRNA levels. RIC significantly increased Nrf2, HO-1, and NQO1 protein expression and decreased Keap1, NLRP3, Cleaved Caspase-1, Cleaved IL-1β, IL-6, and TNF-α protein expression. RIC promoted the activation and translocation of Nrf2 into the nucleus. The protective effects of RIC were abolished by ML385 treatment. In conclusion, our findings suggest that RIC alleviates oxidative stress and inflammatory responses via the Nrf2/HO-1 pathway, which in turn improves neurobehavioral function. RIC may provide novel therapeutic options for acute ischemic stroke.
Keywords: Inflammation; MCAO; Nrf2/HO-1 pathway; Oxidative stress; Remote ischemic conditioning.
Copyright © 2023 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

