Clinical Performance Evaluation of Continuous Glucose Monitoring Systems: A Scoping Review and Recommendations for Reporting
- PMID: 37599389
- PMCID: PMC10658695
- DOI: 10.1177/19322968231190941
Clinical Performance Evaluation of Continuous Glucose Monitoring Systems: A Scoping Review and Recommendations for Reporting
Abstract
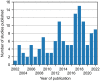
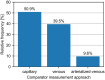
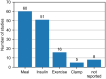
The use of different approaches for design and results presentation of studies for the clinical performance evaluation of continuous glucose monitoring (CGM) systems has long been recognized as a major challenge in comparing their results. However, a comprehensive characterization of the variability in study designs is currently unavailable. This article presents a scoping review of clinical CGM performance evaluations published between 2002 and 2022. Specifically, this review quantifies the prevalence of numerous options associated with various aspects of study design, including subject population, comparator (reference) method selection, testing procedures, and statistical accuracy evaluation. We found that there is a large variability in nearly all of those aspects and, in particular, in the characteristics of the comparator measurements. Furthermore, these characteristics as well as other crucial aspects of study design are often not reported in sufficient detail to allow an informed interpretation of study results. We therefore provide recommendations for reporting the general study design, CGM system use, comparator measurement approach, testing procedures, and data analysis/statistical performance evaluation. Additionally, this review aims to serve as a foundation for the development of a standardized CGM performance evaluation procedure, thereby supporting the goals and objectives of the Working Group on CGM established by the Scientific Division of the International Federation of Clinical Chemistry and Laboratory Medicine.
Keywords: accuracy; clinical performance evaluation; continuous glucose monitoring; study design.
Conflict of interest statement
Declaration of Conflicting InterestsThe author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: G.F. is the general manager and medical director of the Institute for Diabetes Technology (Institut für Diabetes-Technologie Forschungs- und Entwicklungsgesellschaft mbH an der Universität Ulm, Ulm, Germany), which carries out clinical studies, eg, with medical devices for diabetes therapy on its own initiative and on behalf of various companies. G.F./IfDT have received research support, speakers’ honoraria, or consulting fees in the last three years from Abbott, Ascensia, Berlin Chemie, Boydsense, Dexcom, Lilly, Metronom, Medtronic, Menarini, MySugr, Novo Nordisk, PharmaSens, Roche, Sanofi, Terumo. M.E., D.W., S.P., S.W., and C.H. are employees of IfDT. R.S. is the chair of the Clinical Chemistry Department of Isala which carries out clinical studies, eg, with medical devices for diabetes therapy on its own initiative and on behalf of various companies. R.S. has received speakers’ honoraria or consulting fees in the last three years from Roche and Menarini. J.J. has for the last three years been a lecturer/member of the scientific advisory board for Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Medtronic, Nordic InfuCare, NovoNordisk A/S, and Sanofi. R.H. is an employee of Roche Diabetes Care GmbH. L.W. has received funding from the Diabetes Center Berne. E.E.B. and K.M. have no disclosures. D.C.K. is a consultant for Atropos Health, Better Therapeutics, Eoflow, Integrity, Lifecare, Nevro, Novo, Sanofi, and Thirdwayv. N.T. is a consultant for Roche Diagnostics and Radiometer. J.H.N. has received research support from Abbott. P.D. is a board member of PharmaSens. A.T. is a freelance consultant. He has received fees for lectures or consultancy fees from Abbott, Berlin Chemie, Dexcom, Evivamed, Menarini, Novo Nordisk, Roche, and Sanofi in the last three years.
Figures






References
-
- American Diabetes Association. 7. Diabetes technology: standards of medical care in diabetes—2021. Diabetes Care. 2021;44(supplement 1):S85. - PubMed
-
- Clinical and Laboratory Standards Institute (CLSI). Performance Metrics for Continuous Interstitial Glucose Monitoring (CLSI Guideline POCT05). 2nd ed. Wayne, PA: CLSI; 2020

