Perivascular adipose tissue as a source of therapeutic targets and clinical biomarkers
- PMID: 37599464
- PMCID: PMC10568001
- DOI: 10.1093/eurheartj/ehad484
Perivascular adipose tissue as a source of therapeutic targets and clinical biomarkers
Abstract
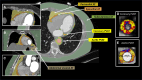
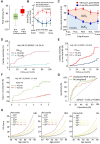
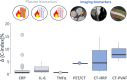
Obesity is a modifiable cardiovascular risk factor, but adipose tissue (AT) depots in humans are anatomically, histologically, and functionally heterogeneous. For example, visceral AT is a pro-atherogenic secretory AT depot, while subcutaneous AT represents a more classical energy storage depot. Perivascular adipose tissue (PVAT) regulates vascular biology via paracrine cross-talk signals. In this position paper, the state-of-the-art knowledge of various AT depots is reviewed providing a consensus definition of PVAT around the coronary arteries, as the AT surrounding the artery up to a distance from its outer wall equal to the luminal diameter of the artery. Special focus is given to the interactions between PVAT and the vascular wall that render PVAT a potential therapeutic target in cardiovascular diseases. This Clinical Consensus Statement also discusses the role of PVAT as a clinically relevant source of diagnostic and prognostic biomarkers of vascular function, which may guide precision medicine in atherosclerosis, hypertension, heart failure, and other cardiovascular diseases. In this article, its role as a 'biosensor' of vascular inflammation is highlighted with description of recent imaging technologies that visualize PVAT in clinical practice, allowing non-invasive quantification of coronary inflammation and the related residual cardiovascular inflammatory risk, guiding deployment of therapeutic interventions. Finally, the current and future clinical applicability of artificial intelligence and machine learning technologies is reviewed that integrate PVAT information into prognostic models to provide clinically meaningful information in primary and secondary prevention.
Keywords: Atherosclerosis; Coronary computed tomography angiography; Fat attenuation index; Peri-vascular adipose tissue.
© The Author(s) 2023. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures




References
-
- NCD Risk Factor Collaboration . Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017;390:2627–42. 10.1016/S0140-6736(17)32129-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

