Expectations, symptoms, and quality of life before and after 1 year of Pirfenidone treatment in patients with idiopathic pulmonary fibrosis: A single-arm, open-label nonrandomized study
- PMID: 37599655
- PMCID: PMC10432581
- DOI: 10.1002/hsr2.1449
Expectations, symptoms, and quality of life before and after 1 year of Pirfenidone treatment in patients with idiopathic pulmonary fibrosis: A single-arm, open-label nonrandomized study
Abstract
Background and aims: Antifibrotic therapies reduce lung function decline in patients with idiopathic pulmonary fibrosis (IPF). This single-arm, open-label, nonrandomized study aimed to determine the influence of antifibrotic treatment on patients' reported symptoms and expectations of the therapy.
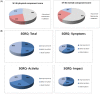
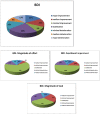
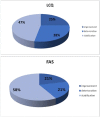
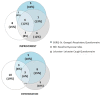
Methods: Fifty-two patients with confirmed IPF at a mean age of 65 ± 8.63 years (73% male) completed the following surveys at baseline and after 12 months of Pirfenidone treatment: Short Form Healthy Survey (SF-36), St. George's Respiratory Questionnaire (SGRQ), Baseline Dyspnea Index (BDI), Fatigue Assessment Scale (FAS), Leicester Cough Questionnaire (LCQ), and Patient's Needs and Expectations Authors' Survey.
Results: The most important patients' needs were access to novel therapy, fast and easy access to health centers specializing in IPF treatment, and the improvement of the general condition or the maintenance of its level. These needs did not change with time, except for the significantly more important right of deciding on disease management after 12 months of treatment (p = 0.014). The quality of life per SF-36, after 1 year of Pirfenidone treatment, significantly improved in the physical cumulative score (p = 0.004) and mental cumulative score (p = 0.003). Significant deteriorations were observed in bodily pain and vitality. For the remaining questionnaires (SGRQ, BDI, FAS, and LCQ), no significant changes in the course of the study were noticed. Around one in 10 patients subjected to Pirfenidone therapy had achieved general symptom improvement in all areas; that is, quality of life improvement as well as cough and dyspnea reduction.
Conclusions: One year of antifibrotic treatment resulted in a general improvement in the quality of life per the SF-36 questionnaire. Patients' expectations of disease management did not change; also, access to novel therapies and easy access to health centers specializing in IPF management remained their top needs.
Keywords: Pirfenidone; antifibrotic therapies; cough; dyspnea; fatigue; idiopathic pulmonary fibrosis; patients' expectations; quality of life.
© 2023 The Authors. Health Science Reports published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Ley B, Collard HR, King TE. Clinical course and prediction of survival in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:431‐440. - PubMed
-
- Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet. 2011;377:1760‐1769. - PubMed
-
- King TE, Bradford WZ, Castro‐Bernardini S, et al. A phase 3 trial of Pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2083‐2092. - PubMed
-
- Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of Nintedanib in idiopathic pulmonary fibrosis. N Engl J Med. 2014;370:2071‐2082. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

