Chemical Tools to Image the Activity of PAR-Cleaving Proteases
- PMID: 37599791
- PMCID: PMC10436261
- DOI: 10.1021/acsbiomedchemau.3c00019
Chemical Tools to Image the Activity of PAR-Cleaving Proteases
Abstract
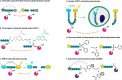
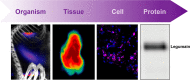
Protease-activated receptors (PARs) comprise a family of four G protein-coupled receptors (GPCRs) that have broad functions in health and disease. Unlike most GPCRs, PARs are uniquely activated by proteolytic cleavage of their extracellular N termini. To fully understand PAR activation and function in vivo, it is critical to also study the proteases that activate them. As proteases are heavily regulated at the post-translational level, measures of total protease abundance have limited utility. Measures of protease activity are instead required to inform their function. This review will introduce several classes of chemical probes that have been developed to measure the activation of PAR-cleaving proteases. Their strengths, weaknesses, and applications will be discussed, especially as applied to image protease activity at the whole organism, tissue, and cellular level.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

