Thermocatalytic Decomposition of Methane: A Review on Carbon-Based Catalysts
- PMID: 37599913
- PMCID: PMC10433352
- DOI: 10.1021/acsomega.3c01936
Thermocatalytic Decomposition of Methane: A Review on Carbon-Based Catalysts
Abstract
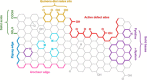
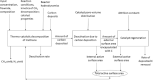
The global initiatives on sustainable and green energy resources as well as large methane reserves have encouraged more research to convert methane to hydrogen. Catalytic decomposition of methane (CDM) is one optimistic route to generate clean hydrogen and value-added carbon without the emission of harmful greenhouse gases, typically known as blue hydrogen. This Review begins with an attempt to understand fundamentals of a CDM process in terms of thermodynamics and the prerequisite characteristics of the catalyst materials. In-depth understanding of rate-determining steps of the heterogeneous catalytic reaction taking place over the catalyst surfaces is crucial for the development of novel catalysts and process conditions for a successful CDM process. The design of state-of-the-art catalysts through both computational and experimental optimizations is the need of hour, as it largely governs the economy of the process. Recent mono- and bimetallic supported and unsupported materials used in CDM process have been highlighted and classified based on their performances under specific reaction conditions, with an understanding of their advantages and limitations. Metal oxides and zeolites have shown interesting performance as support materials for Fe- and Ni-based catalysts, especially in the presence of promoters, by developing strong metal-support interactions or by enhancing the carbon diffusion rates. Carbonaceous catalysts exhibit lower conversions without metal active species and largely result in the formation of amorphous carbon. However, the stability of carbon catalysts is better than that of metal oxides at higher temperatures, and the overall performance depends on the operating conditions, catalyst properties, and reactor configurations. Although efforts to summarize the state-of-art have been reported in literature, they lack systematic analysis on the development of stable and commercially appealing CDM technology. In this work, carbon catalysts are seen as promising futuristic pathways for sustained H2 production and high yields of value-added carbon nanomaterials. The influence of the carbon source, particle size, surface area, and active sites on the activity of carbon materials as catalysts and support templates has been demonstrated. Additionally, the catalyst deactivation process has been discussed, and different regeneration techniques have been evaluated. Recent studies on theoretical models towards better performance have been summarized, and future prospects for novel CDM catalyst development have been recommended.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

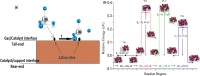
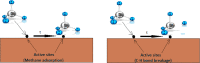


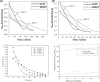

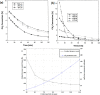
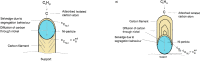
References
-
- Pudukudy M.; Yaakob Z. Methane decomposition over Ni, Co and Fe based monometallic catalysts supported on sol gel derived SiO2 microflakes. Chemical Engineering Journal. 2015, 262, 1009–1021. 10.1016/j.cej.2014.10.077. - DOI
-
- Neeli S. T.; Ramsurn H. Synthesis and formation mechanism of iron nanoparticles in graphitized carbon matrix using biochar from biomass model compounds as a support. Carbon. 2018, 134, 480–490. 10.1016/j.carbon.2018.03.079. - DOI
-
- Wang I. W.; Kutteri D. A.; Gao B.; Tian H.; Hu J. Methane pyrolysis for carbon nanotubes and COx -free H2 over transition-metal catalysts. Energy and Fuels. 2019, 33, 197–205. 10.1021/acs.energyfuels.8b03502. - DOI
-
- Hamdani I. R.; Bhaskarwar A. N. Cu2O nanowires based p-n homojunction photocathode for improved current density and hydrogen generation through solar-water splitting. Int. J. Hydrogen Energy. 2021, 46, 28064–28077. 10.1016/j.ijhydene.2021.06.067. - DOI
-
- Hamdani I. R.; Bhaskarwar A. N. Tuning of the structural, morphological, optoelectronic and interfacial properties of electrodeposited Cu2O towards solar water-splitting by varying the deposition pH. Solar Energy Materials and Solar Cells. 2022, 240, 111719.10.1016/j.solmat.2022.111719. - DOI
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

