Differential CRH expression level determines efficiency of Cre- and Flp-dependent recombination
- PMID: 37599997
- PMCID: PMC10434532
- DOI: 10.3389/fnins.2023.1163462
Differential CRH expression level determines efficiency of Cre- and Flp-dependent recombination
Abstract
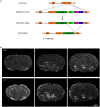
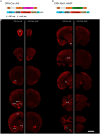
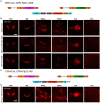
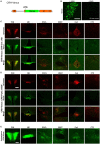
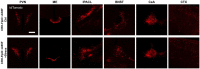
Corticotropin-releasing hormone expressing (CRH+) neurons are distributed throughout the brain and play a crucial role in shaping the stress responses. Mouse models expressing site-specific recombinases (SSRs) or reporter genes are important tools providing genetic access to defined cell types and have been widely used to address CRH+ neurons and connected brain circuits. Here, we investigated a recently generated CRH-FlpO driver line expanding the CRH system-related tool box. We directly compared it to a previously established and widely used CRH-Cre line with respect to the FlpO expression pattern and recombination efficiency. In the brain, FlpO mRNA distribution fully recapitulates the expression pattern of endogenous Crh. Combining both Crh locus driven SSRs driver lines with appropriate reporters revealed an overall coherence of respective spatial patterns of reporter gene activation validating CRH-FlpO mice as a valuable tool complementing existing CRH-Cre and reporter lines. However, a substantially lower number of reporter-expressing neurons was discerned in CRH-FlpO mice. Using an additional CRH reporter mouse line (CRH-Venus) and a mouse line allowing for conversion of Cre into FlpO activity (CAG-LSL-FlpO) in combination with intersectional and subtractive mouse genetic approaches, we were able to demonstrate that the reduced number of tdTomato reporter expressing CRH+ neurons can be ascribed to the lower recombination efficiency of FlpO compared to Cre recombinase. This discrepancy particularly manifests under conditions of low CRH expression and can be overcome by utilizing homozygous CRH-FlpO mice. These findings have direct experimental implications which have to be carefully considered when targeting CRH+ neurons using CRH-FlpO mice. However, the lower FlpO-dependent recombination efficiency also entails advantages as it provides a broader dynamic range of expression allowing for the visualization of cells showing stress-induced CRH expression which is not detectable in highly sensitive CRH-Cre mice as Cre-mediated recombination has largely been completed in all cells generally possessing the capacity to express CRH. These findings underscore the importance of a comprehensive evaluation of novel SSR driver lines prior to their application.
Keywords: CRF; CRH; Cre; Flp; corticotropin-releasing hormone; reporter; stress.
Copyright © 2023 Zhao, Ries, Du, Zhang, Sakimura, Itoi and Deussing.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

