Mid-term changes in cognitive functions in patients with atrial fibrillation: a longitudinal analysis of the Swiss-AF cohort
- PMID: 37600058
- PMCID: PMC10433225
- DOI: 10.3389/fcvm.2023.1212587
Mid-term changes in cognitive functions in patients with atrial fibrillation: a longitudinal analysis of the Swiss-AF cohort
Abstract
Background: Longitudinal association studies of atrial fibrillation (AF) and cognitive functions have shown an unclear role of AF-type and often differ in methodological aspects. We therefore aim to investigate longitudinal changes in cognitive functions in association with AF-type (non-paroxysmal vs. paroxysmal) and comorbidities in the Swiss-AF cohort.
Methods: Seven cognitive measures were administered up to five times between 2014 and 2022. Age-education standardized scores were calculated and association between longitudinal change in scores and baseline AF-type investigated using linear mixed-effects models. Associations between AF-type and time to cognitive drop, an observed score of at least one standard deviation below individual's age-education standardized cognitive scores at baseline, were studied using Cox proportional hazard models of each cognitive test, censoring patients at their last measurement. Models were adjusted for baseline covariates.
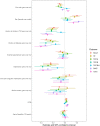
Results: 2,415 AF patients (mean age 73.2 years; 1,080 paroxysmal, 1,335 non-paroxysmal AF) participated in this Swiss multicenter prospective cohort study. Mean cognitive scores increased longitudinally (median follow-up 3.97 years). Non-paroxysmal AF patients showed smaller longitudinal increases in Digit Symbol Substitution Test (DSST), Cognitive Construct Score (CoCo)and Trail Making Test part B (TMT-B) scores vs. paroxysmal AF patients. Diabetes, history of stroke/TIA and depression were associated with worse performance on all cognitive tests. No differences in time to cognitive drop were observed between AF-types in any cognitive test.
Conclusion: This study indicated preserved cognitive functioning in AF patients, best explained by practice effects. Smaller practice effects were found in non-paroxysmal AF patients in the DSST, TMT-B and the CoCo and could indicate a marker of subtle cognitive decline. As diabetes, history of stroke/TIA and depression-but not AF-type-were associated with cognitive drop, more attention should be given to risk factors and underlying mechanisms of AF.
Keywords: Swiss-AF; atrial fibrillation; cognitive function; longitudinal cohort study; practice effect.
© 2023 Wueest, Zuber, Coslovsky, Rommers, Rodondi, Gencer, Moschovitis, De Perna, Beer, Reichlin, Krisai, Springer, Conen, Stauber, Mueller, Paladini, Kuhne, Osswald, Monsch and Bonati.
Conflict of interest statement
NR received a grant from the Swiss Heart Foundation. GM has received consultant fees for taking part to advisory boards from Astra Zeneca, Bayer, Boehringer Ingelheim, Daiichi Sankyo, Gebro Pharma, Novartis and Vifor, all outside of the submitted work. JB reports grants from the Swiss National Foundation of Science, the Swiss Heart Foundation, the Kardio foundation and received grant support and consultancy fees to the institution from Bayer, Sanofi, and Daitchii. TR reveived consulting honoraria or travel support from Abbott/SJM, Astra Zeneca, Brahms, Bayer, Biosense-Webster, Medtronic, Pfizer-BMS and Roche, all outside of the presented work. He reports research grants from the Swiss National Science Foundation and the Swiss Heart Foundation, speaker/consulting honoraria or travel support from Abbott/SJM, Astra Zeneca, Brahms, Bayer, Biosense-Webster, Biotronik, Boston-Scientific, Daiichi Sankyo, Medtronic, Pfizer-BMS and Roche, support for his institution's fellowship program from Abbott/SJM, Biosense-Webster, Biotronik, Boston-Scientific and Medtronic. PK received speaker fee from BMS/Pfizer. DC has received advisory board fees from Roche Diagnostics and Trimedics; speaker fees from BMS/Pfizer and Servier. ASM has received fellowship and training support from Biotronik, Boston Scientific, Medtronic, Abbott/St. Jude Medical, and Biosense Webster, and speaker honoraria from Biosense Webster, Medtronic, Abbott/St. Jude Medical, AstraZeneca, Daiichi Sankyo, Biotronik, MicroPort and is a consultant for Biosense Webster, Medtronic, and Abbott/St. Jude Medical. KM reports grants from Bayer, grants from Pfizer, grants from Boston Scientific, grants from BMS, grants and personal fees from Daiichi Sankyo. SO received research grant from Swiss National Science Foundation (SNSF) for Swiss-AF Cohort study (33CS30_18474/1&2), research grant from Swiss National Science Foundation (SNSF) for Swiss-AF Control study (324730_192394/1), research grants from Swiss Heart Foundation (SHS), research grants from Foundation for Cardio Vascular Research Basel (SKFB), research grants from Roche, educational and Speaker Office grants from Roche, Bayer, Novartis, Sanofi AstraZeneca, Daiichi-Sankyo, Pfizer. AM received honoraria and/or research support from AC Immune, Biogen, Bristol Myers Squibb, DSM, GlaxoSmithKline, Janssen, Lundbeck, Merz Pharma, Novartis, OM Pharma, Pfizer, Roche, and Schwabe, all outside of the submitted work. LB received grants from the Swiss National Science Foundation (PBBSB-116873, 33CM30-124119, 32003B-156658, 32003B-197524; Berne, Switzerland), The Swiss Heart Foundation (Berne, Switzerland; and the University of Basel (Basel, Switzerland), unrestricted research grant from AstraZeneca, consultancy or advisory board fees or speaker's honoraria from Amgen, Bayer, Bristol-Myers Squibb, and Claret Medical, and travel grants from AstraZeneca and Bayer. The other remaining authors declare no conflict of interest. All financial, commercial or other relationships that might be perceived by the academic community as representing a potential conflict of interest must be disclosed.
Figures



References
LinkOut - more resources
Full Text Sources
Research Materials

