The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases
- PMID: 37600764
- PMCID: PMC10437113
- DOI: 10.3389/fimmu.2023.1191782
The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases
Erratum in
-
Corrigendum: The paradigm of IL-23-independent production of IL-17F and IL-17A and their role in chronic inflammatory diseases.Front Immunol. 2023 Nov 22;14:1332177. doi: 10.3389/fimmu.2023.1332177. eCollection 2023. Front Immunol. 2023. PMID: 38077344 Free PMC article.
Abstract
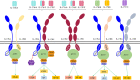
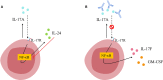
Interleukin-17 family (IL-17s) comprises six structurally related members (IL-17A to IL-17F); sequence homology is highest between IL-17A and IL-17F, displaying certain overlapping functions. In general, IL-17A and IL-17F play important roles in chronic inflammation and autoimmunity, controlling bacterial and fungal infections, and signaling mainly through activation of the nuclear factor-kappa B (NF-κB) pathway. The role of IL-17A and IL-17F has been established in chronic immune-mediated inflammatory diseases (IMIDs), such as psoriasis (PsO), psoriatic arthritis (PsA), axial spondylarthritis (axSpA), hidradenitis suppurativa (HS), inflammatory bowel disease (IBD), multiple sclerosis (MS), and asthma. CD4+ helper T cells (Th17) activated by IL-23 are well-studied sources of IL-17A and IL-17F. However, other cellular subtypes can also produce IL-17A and IL-17F, including gamma delta (γδ) T cells, alpha beta (αβ) T cells, type 3 innate lymphoid cells (ILC3), natural killer T cells (NKT), or mucosal associated invariant T cells (MAIT). Interestingly, the production of IL-17A and IL-17F by innate and innate-like lymphocytes can take place in an IL-23 independent manner in addition to IL-23 classical pathway. This would explain the limitations of the inhibition of IL-23 in the treatment of patients with certain rheumatic immune-mediated conditions such as axSpA. Despite their coincident functions, IL-17A and IL-17F contribute independently to chronic tissue inflammation having somehow non-redundant roles. Although IL-17A has been more widely studied, both IL-17A and IL-17F are overexpressed in PsO, PsA, axSpA and HS. Therefore, dual inhibition of IL-17A and IL-17F could provide better outcomes than IL-23 or IL-17A blockade.
Keywords: IL-17A; IL-17F; IL-23; MAIT cells; Th17 cells; psoriasis; spondyloarthritis; γδ T cells.
Copyright © 2023 Navarro-Compán, Puig, Vidal, Ramírez, Llamas-Velasco, Fernández-Carballido, Almodóvar, Pinto, Galíndez-Aguirregoikoa, Zarco, Joven, Gratacós, Juanola, Blanco, Arias-Santiago, Sanz Sanz, Queiro and Cañete.
Conflict of interest statement
Author VN-C has served as a speaker, consultant, and/or instructor for: AbbVie, Eli Lilly and Company, Galapagos, Janssen, Moonlake, Novartis, Pfizer, and UCB Pharma; and has received grant and/or research support from AbbVie and Novartis. Author LP has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Sandoz, Sanofi, and UCB. Author SV has received speaker’s honoraria and participated in projects sponsored by Bayer, Janssen, LEO Pharma, Lilly, Novartis, Pfizer, Roche, Sanofi and UCB. Author JR has received consultancy and/or speaker’s honoraria from Abbvie, UCB, Janssen, Novartis, Pfizer, Amgen and Lilly and/or participated in clinical trials and/or research projects sponsored by Pfizer, Novartis and Janssen. Author ML-V has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Janssen, Kyowa Kirian, LEO Pharma, Lilly, Novartis, and UCB. Author CF-C has received consultancy and/or speaker’s honoraria and participated in clinical trials and/or research projects sponsored by AbbVie, Janssen, Lilly, MSD, Novartis, Pfizer, the Spanish Society of Rheumatology and UCB. Author RA has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, Almirall, Amgen, Galápagos, Gebro, Janssen, Lilly, MSD, Nordimet, Novartis, Pfizer and UCB. Author JP has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by Janssen, Novartis, Pfizer, MSD, Lilly, Amgen, BMS, AbbVie, and UCB. Author EG-A has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, MSD, Roche, Amgen, Janssen, Lilly, Novartis, Pfizer and UCB. Author PZ has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, Celgene, Galapagos, Janssen, Lilly, MSD, Novartis, Pfizer and UCB. Author BJ has received consultancy fees from Amgen, UCB and Janssen; has received speaker’s honoraria from Lilly, Abbvie and Janssen; has participated in clinical trials and/or research projects sponsored by Janssen, Lilly, Bristol Myers Squibb, Abbvie; has received support for attending congress from Novartis, Pfizer, UCB. Author JG has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by Novartis, UCB, Pfizer, BMS, MSD, AbbVie, Lilly, Janssen, AstraZeneca and Galápagos. Author XJ has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, Lilly, MSD, Nordic Pharma, Novartis, Pfizer and UCB. Author RB has received grants/research supports from Abbvie, MSD, and Roche, and had consultation fees/participation in company-sponsored speaker´s bureau from Abbvie, Pfizer, Roche, Bristol-Myers, Lilly, Janssen, and MSD. Author SA has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, Almirall, Janssen, LEO Pharma, Lilly, Novartis and UCB. Author JS has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, UCB, Novartis, Amgen, Pfizer and Janssen-Cilag. Author RQ has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by Novartis, Janssen, UCB, Pfizer, Amgen, MSD, Eli-Lilly and AbbVie. Author JC has received consultancy and/or speaker’s honoraria from and/or participated in clinical trials and/or research projects sponsored by AbbVie, Almirall, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Fresenius, Janssen, Lilly, Novartis, Pfizer, Sandoz and UCB.
Figures

References
-
- Ruiz de Morales JMG, Puig L, Dauden E, Canete JD, Pablos JL, Martin AO, et al. . Critical role of interleukin (IL)-17 in inflammatory and immune disorders: An updated review of the evidence focusing in controversies. Autoimmun Rev (2020) 19(1):102429. doi: 10.1016/j.autrev.2019.102429 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

