Polyfunctional antibodies: a path towards precision vaccines for vulnerable populations
- PMID: 37600816
- PMCID: PMC10433199
- DOI: 10.3389/fimmu.2023.1183727
Polyfunctional antibodies: a path towards precision vaccines for vulnerable populations
Abstract
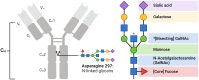
Vaccine efficacy determined within the controlled environment of a clinical trial is usually substantially greater than real-world vaccine effectiveness. Typically, this results from reduced protection of immunologically vulnerable populations, such as children, elderly individuals and people with chronic comorbidities. Consequently, these high-risk groups are frequently recommended tailored immunisation schedules to boost responses. In addition, diverse groups of healthy adults may also be variably protected by the same vaccine regimen. Current population-based vaccination strategies that consider basic clinical parameters offer a glimpse into what may be achievable if more nuanced aspects of the immune response are considered in vaccine design. To date, vaccine development has been largely empirical. However, next-generation approaches require more rational strategies. We foresee a generation of precision vaccines that consider the mechanistic basis of vaccine response variations associated with both immunogenetic and baseline health differences. Recent efforts have highlighted the importance of balanced and diverse extra-neutralising antibody functions for vaccine-induced protection. However, in immunologically vulnerable populations, significant modulation of polyfunctional antibody responses that mediate both neutralisation and effector functions has been observed. Here, we review the current understanding of key genetic and inflammatory modulators of antibody polyfunctionality that affect vaccination outcomes and consider how this knowledge may be harnessed to tailor vaccine design for improved public health.
Keywords: Fc function; Fc receptor; IgG glycosylation; allotype; antibody; computational modelling; polymorphism; vaccine design.
Copyright © 2023 Purcell, Theisen, Arnold, Chung and Selva.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

