Apoptotic cells for treatment of acute respiratory distress syndrome associated with COVID-19
- PMID: 37600829
- PMCID: PMC10433372
- DOI: 10.3389/fimmu.2023.1242551
Apoptotic cells for treatment of acute respiratory distress syndrome associated with COVID-19
Abstract
Background: Hyper-inflammatory immune response, a hallmark of severe COVID-19, is associated with increased mortality. Acute respiratory distress syndrome (ARDS) is a common manifestation. We undertook two phase I/II studies in five and then 16 subjects with severe/critical COVID-19 to assess the safety and preliminary efficacy of apoptotic cells (Allocetra™-OTS, Enlivex Therapeutics), a cellular immunomodulatory therapy that reprograms macrophages to reduce hyper-inflammatory response severity.
Methods: Eligible patients presenting to the Emergency Room with severe COVID-19 and respiratory dysfunction received one intravenous administration of Allocetra™-OTS and were monitored for adverse events (AEs) for 28 days. The primary aim was to determine the safety profile of treatment; secondary aims were recovery from ARDS, intensive care unit (ICU) and hospital length-of-stay, and mortality. Immune modulator markers were measured to elucidate the mechanism of action of Allocetra™-OTS.
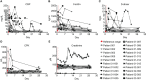
Results: 21 patients with severe-critical COVID-19 of Gamma, Alpha and Delta variants, were treated with a single dose of apoptotic cells. 19/21 patients had mild-to-severe ARDS at presentation. Median age was 53 years, 16/21 were males, 16/21 were overweight/obese. No serious related adverse events (SAEs) were reported. All 21 study subjects survived to day 28 (end of study); 19/21 recovered completely. Comparable mortality rates at the hospital were 3.8%-8.9% for age- and gender-matched patients, and 39%-55% for critical patients. Recovering patients exhibited rapid ARDS resolution and parallel resolution of inflammation markers and elevated cytokines/chemokines.
Conclusion: In patients with severe/critical COVID-19 associated with ARDS, Allocetra™-OTS was safe, well-tolerated, and showed promising results for resolution of respiratory failure and inflammation.
Trial registration: https://clinicaltrials.gov/ct2/show/study/NCT04513470, https://clinicaltrials.gov/ct2/show/study/NCT04590053, Identifiers NCT04513470, NCT04590053.
Keywords: ARDS; COVID-19; apoptotic cells; cytokine storm; macrophage reprogramming.
Copyright © 2023 van Heerden, Abutbul, Naama, Maayan, Makram, Nachshon, abu Jabal, Hershkovitz, Binder, Shabat, Reicher and Mevorach.
Conflict of interest statement
PH received honoraria from Enlivex Ltd as a consultant. DM is the founder and the Chief Scientific Officer of Enlivex Therapeutics Ltd. YS, BR, LB and OH are employed by Enlivex Therapeutics Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







References
-
- Mathieu E, Ritchie H, Rodés-Guirao L, Appel C, Giattino G, Hasell J, et al. Coronovirus Pandemic (COVID-19) (2020). Available at: https://ourworldindata.org/coronavirus.
-
- Group ICC, Kartsonaki C. Characteristics and outcomes of an international cohort of 400,000 hospitalised patients with Covid-19. medRxiv (2021).
-
- Carbonell R, Urgeles S, Rodriguez A, Bodi M, Martin-Loeches I, Sole-Violan J, et al. Mortality comparison between the first and second/third waves among 3,795 critical COVID-19 patients with pneumonia admitted to the ICU: A multicentre retrospective cohort study. Lancet Reg Health Eur (2021) 11:100243. doi: 10.1016/j.lanepe.2021.100243 - DOI - PMC - PubMed
-
- Dongelmans DA, Termorshuizen F, Brinkman S, Bakhshi-Raiez F, Arbous MS, de Lange DW, et al. Characteristics and outcome of COVID-19 patients admitted to the ICU: a nationwide cohort study on the comparison between the first and the consecutive upsurges of the second wave of the COVID-19 pandemic in the Netherlands. Ann Inten Care (2022) 12(1):5. doi: 10.1186/s13613-021-00978-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Supplementary concepts
Associated data
LinkOut - more resources
Full Text Sources
Medical

