Evaluation of the biofilm-forming ability and molecular characterization of dairy Bacillus spp. isolates
- PMID: 37600945
- PMCID: PMC10432688
- DOI: 10.3389/fcimb.2023.1229460
Evaluation of the biofilm-forming ability and molecular characterization of dairy Bacillus spp. isolates
Abstract
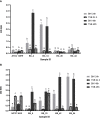
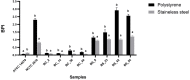
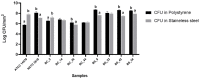
Food processing lines represents a suitable environment for bacterial biofilm formation. One of the most common biofilm-forming genera in dairy processing plants is Bacillus, which includes species that may have a negative impact on safety and/or quality of dairy products. In the current study, we evaluated the biofilm forming ability and molecular characteristics of dairy Bacillus spp. isolates (B. cereus and B. subtilis). Reference strains (B. cereus ATCC 14579 and B. subtilis NCTC 3610) were also included in the experiment. All isolates were screened by micro-titer plate (96 wells) to assess their ability to form biofilm. Then, they were tested on two common food contact surfaces (polystyrene and stainless steel) by using 6-well plates and AISI 316 stainless steel coupons. Biofilm formation, expressed as biofilm production index (BPI), was higher on polystyrene than stainless steel (except for B. cereus ATCC 14579). These observations were further confirmed by scanning electron microscopy, which allowed the microscopy observation of biofilm structure. Moreover, a possible correlation among total viable cell counts (CFU) and BPI was examined, as well as a connection among biofilm formation and bacterial cell hydrophobicity. Finally, whole genome sequencing was performed highlighting a genetic similarity among the strains belonging to the same species. The presence of selected genes involved in biofilm formation was also examined showing that strains with a greater presence of these genes were able to produce more biofilm in the tested materials. Additionally, for B. cereus strains enterotoxin genes were detected.
Keywords: Bacillus; SEM; biofilm; polystyrene; stainless-steel; whole genome sequencing.
Copyright © 2023 Catania, Di Ciccio, Ferrocino, Civera, Cannizzo and Dalmasso.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Effect of gaseous ozone treatment on cells and biofilm of dairy Bacillus spp. isolates.Front Microbiol. 2025 Mar 17;16:1538456. doi: 10.3389/fmicb.2025.1538456. eCollection 2025. Front Microbiol. 2025. PMID: 40165788 Free PMC article.
-
Comparative analysis of biofilm formation by Bacillus cereus reference strains and undomesticated food isolates and the effect of free iron.Int J Food Microbiol. 2015 May 4;200:72-9. doi: 10.1016/j.ijfoodmicro.2015.02.005. Epub 2015 Feb 12. Int J Food Microbiol. 2015. PMID: 25700364
-
Biofilm formation potential of Bacillus toyonensis and Pseudomonas aeruginosa on the stainless steel test surfaces in a model dairy batch system.Folia Microbiol (Praha). 2022 Jun;67(3):405-417. doi: 10.1007/s12223-021-00940-7. Epub 2022 Jan 15. Folia Microbiol (Praha). 2022. PMID: 35031974
-
Prevalence and expression of enterotoxins in Bacillus cereus and other Bacillus spp., a literature review.Antonie Van Leeuwenhoek. 2000 May;77(4):393-9. doi: 10.1023/a:1002706906154. Antonie Van Leeuwenhoek. 2000. PMID: 10959569 Review.
-
Bacillus cereus Biofilms-Same, Only Different.Front Microbiol. 2016 Jul 7;7:1054. doi: 10.3389/fmicb.2016.01054. eCollection 2016. Front Microbiol. 2016. PMID: 27458448 Free PMC article. Review.
Cited by
-
Effect of gaseous ozone treatment on cells and biofilm of dairy Bacillus spp. isolates.Front Microbiol. 2025 Mar 17;16:1538456. doi: 10.3389/fmicb.2025.1538456. eCollection 2025. Front Microbiol. 2025. PMID: 40165788 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

