A review of a strategic roadmapping exercise to advance clinical translation of photoacoustic imaging: From current barriers to future adoption
- PMID: 37600964
- PMCID: PMC10432856
- DOI: 10.1016/j.pacs.2023.100539
A review of a strategic roadmapping exercise to advance clinical translation of photoacoustic imaging: From current barriers to future adoption
Abstract
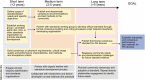
Photoacoustic imaging (PAI), also referred to as optoacoustic imaging, has shown promise in early-stage clinical trials in a range of applications from inflammatory diseases to cancer. While the first PAI systems have recently received regulatory approvals, successful adoption of PAI technology into healthcare systems for clinical decision making must still overcome a range of barriers, from education and training to data acquisition and interpretation. The International Photoacoustic Standardisation Consortium (IPASC) undertook an community exercise in 2022 to identify and understand these barriers, then develop a roadmap of strategic plans to address them. Here, we outline the nature and scope of the barriers that were identified, along with short-, medium- and long-term community efforts required to overcome them, both within and beyond the IPASC group.
Keywords: Clinical translation; Optoacoustic tomography; Phantoms; Photoacoustic imaging; Quality assurance; Standardisation.
© 2023 Published by Elsevier GmbH.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: The promotions and salary of Kurinchi Gurusamy are dependent upon journal publications. Ferdinand Knieling is co-inventor with iThera Medical GmbH, Germany on an EU patent (EP 19 163 304.9) relating to a device and a method for analysing optoacoustic data, an optoacoustic system and a computer program. He received travel support by iThera Medical GmbH, Germany, lecture fees from Sanofi Genzyme and Siemens Healthcare GmbH outside the submitted work. Given his role as Section Editor of this journal, Ferdinand Knieling had no involvement in the peer-review of articles for which he was an author and had no access to information regarding their peer-review. AAO is an employee and stock holder of TomoWave and Seno Medical, which develop and sell optoacoustic tomography systems. Given his role as the Editor in Chief of this Journal, AAO had no involvement in the peer-review process or the decisions regarding acceptance of manuscripts for which he is an author. Given her role as an Editorial Board member of this journal, Sarah Bohndiek had no involvement in the peer-review of articles for which she was an author and had no access to information regarding their peer-review. Full responsibility for the peer-review process for this article was delegated to another Editor. Martin Leahy is the inventor of WIPO patent (WO2015110349A1) relating to photoacoustic tomography method and system which uses known intrinsic absorbers for fluence correction. Lihong Wang has a financial interest in Microphotoacoustics, Inc., CalPACT, LLC, and Union Photoacoustic Technologies, Ltd., which, however, did not support this work. Ledia Lilaj is an employee of iThera Medical GmbH, a vendor of optoacoustic imaging instruments. Mithun Kuniyil Ajith Singh is an employee of CYBERDYNE Inc, a vendor of photoacoustic imaging instruments. The other authors do not declare any conflicts of interest.
Figures





References
-
- Wang, L. v & Hu, S. Photoacoustic Tomography: In Vivo Imaging from Organelles to Organs. 〈https://www.science.org〉. - PMC - PubMed
-
- Oraevsky A.A., Karabutov A.A. In: Biomedical Photonics Handbook. Vo-Dinh T., editor. CRC Press; 2003. Optoacoustic tomography.
Publication types
LinkOut - more resources
Full Text Sources
Medical

