Crosstalk between Wnt/β-catenin signaling pathway and DNA damage response in cancer: a new direction for overcoming therapy resistance
- PMID: 37601042
- PMCID: PMC10433774
- DOI: 10.3389/fphar.2023.1230822
Crosstalk between Wnt/β-catenin signaling pathway and DNA damage response in cancer: a new direction for overcoming therapy resistance
Abstract
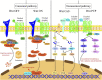
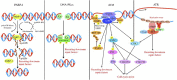
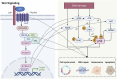
Wnt signaling plays an important role in regulating the biological behavior of cancers, and many drugs targeting this signaling have been developed. Recently, a series of research have revealed that Wnt signaling could regulate DNA damage response (DDR) which is crucial for maintaining the genomic integrity in cells and closely related to cancer genome instability. Many drugs have been developed to target DNA damage response in cancers. Notably, different components of the Wnt and DDR pathways are involved in crosstalk, forming a complex regulatory network and providing new opportunities for cancer therapy. Here, we provide a brief overview of Wnt signaling and DDR in the field of cancer research and review the interactions between these two pathways. Finally, we also discuss the possibility of therapeutic agents targeting Wnt and DDR as potential cancer treatment strategies.
Keywords: DNA damage response; Wnt; cancer; drug resistance; therapy.
Copyright © 2023 Zhang and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources

