Efficacy and safety of total glucosides of paeony in the treatment of recurrent aphthous ulcers: a single-center, double-blind, randomized, placebo-controlled clinical trial
- PMID: 37601076
- PMCID: PMC10437069
- DOI: 10.3389/fphar.2023.1209075
Efficacy and safety of total glucosides of paeony in the treatment of recurrent aphthous ulcers: a single-center, double-blind, randomized, placebo-controlled clinical trial
Abstract
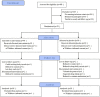
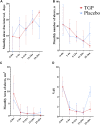
Introduction: There has been a lack of treatments available to lower the frequency of recurrent aphthous ulcers (RAUs) until now. Total glucosides of paeony (TGP) is a botanical drug extracted from the dried roots of Paeonia lactiflora Pall. [Ranunculaceae; Paeoniae Radix Alba]. This study aims to evaluate the efficacy and safety of TGP in the treatment of RAU. Methods: This study was registered with the Chinese Clinical Trial Registry (ChiCTR1900025623). Patients were randomly assigned to the TGP or placebo group and treated with 1.8 g/day for 24 weeks. Participants were observed for a total of 36 weeks and were asked to record ulcer severity, medication, and adverse reactions in the form of diaries or apps every day. The primary outcome was the monthly ulcer-free interval. Results: A total of 79 individuals were enrolled, with 40 assigned to the TGP group and 39 to the placebo group. The dropout rate was 18.18%. In the TGP group, the monthly ulcer-free interval was significantly longer than baseline (median, 9.6 days) since weeks 13-24 (median, 18.5 days) (p < 0.05), and after discontinuation, it was further prolonged (median, 24.7 days) than in weeks 13-24 (p < 0.05). In addition, the monthly ulcer-free interval was longer in the TGP group than in the placebo group (median, 15.9 days) at weeks 25-36 (p < 0.001). There were better improvements in the monthly number of ulcers and monthly area of ulcers, and visual analog scoring in the TGP group at weeks 25-36 (p < 0.001). Conclusion: TGP had a good long-term therapeutic effect on RAU with frequent occurrence. Systematic Review Registration: www.chictr.org.cn, identifier ChiCTR1900025623.
Keywords: Paeonia lactiflora Pall.; efficacy; randomized controlled trial; recurrent aphthous ulcers; safety article types clinical trial; total glucosides of paeony.
Copyright © 2023 Liu, Guo, Li, Lu, Guo, Liu, Wang, Han and Liu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Bratel J., Hakeberg M. (2014). Anamnestic findings from patients with recurrent aphthous stomatitis. Swed. Dent. J. 38 (3), 143–149. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

