Growth, tolerance and safety outcomes with use of an extensively hydrolyzed casein-based formula in infants with cow's milk protein allergy
- PMID: 37601126
- PMCID: PMC10433168
- DOI: 10.3389/fped.2023.1230905
Growth, tolerance and safety outcomes with use of an extensively hydrolyzed casein-based formula in infants with cow's milk protein allergy
Abstract
Objective: To evaluate growth, tolerance and safety outcomes with use of an extensively hydrolyzed casein-based formula (eHCF) in infants with cow's milk protein allergy (CMPA).
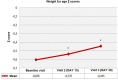
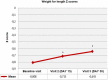
Methods: A total of 226 infants (mean ± SD age: 106.5 ± 39.5 days, 52.7% were girls) with CMPA who received eHCF comprising at least half of the daily dietary intake were included. Data on anthropometrics [weight for age (WFA), length for age (LFA) and weight for length (WFL) z-scores] were recorded at baseline (visit 1), while data on infant feeding and stool records, anthropometrics and Infant Feeding and Stool Patterns and Formula Satisfaction Questionnaires were recorded at visit 2 (on Days 15 ± 5) and visit 3 (on Days 30 ± 5).
Results: From baseline to visit 2 and visit 3, WFA z-scores (from -0.60 ± 1.13 to -0.54 ± 1.09 at visit 2, and to -0.44 ± 1.05 at visit 3, p < 0.001) and WFL z-scores (from -0.80 ± 1.30 to -0.71 ± 1.22 at visit 2, and to -0.64 ± 1.13 at visit 3, p = 0.002) were significantly increased. At least half of infants never experienced irritability or feeding refusal (55.7%) and spit-up after feeding (50.2%). The majority of mothers were satisfied with the study formula (93.2%), and wished to continue using it (92.2%).
Conclusions: In conclusion, eHCF was well-accepted and tolerated by an intended use population of infants ≤ 6 months of age with CMPA and enabled adequate volume consumption and improved growth indices within 30 days of utilization alongside a favorable gastrointestinal tolerance and a high level of parental satisfaction.
Keywords: cow’s milk protein allergy; extensively hydrolyzed casein-based formula; gastrointestinal intolerance; growth indices; parental satisfaction; stool patterns.
© 2023 Kansu, Urganci, Bukulmez, Kutluk, Gulcu Taskin, Sahin Keskin, Igde, Molon, Dogan, Sekerel, Yuksek, Bostanci, Gerenli, Polat, Dalgic, Ayyildiz, Usta, Basturk, Yuce Kirmemis, Tuna Kirsaclioglu, Gulerman, Alptekin Sarioglu and Erdogan.
Conflict of interest statement
AS and SE are Abbott employees. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources