Analytical performance of the FDA-cleared Parsortix® PC1 system
- PMID: 37601320
- PMCID: PMC10434983
- DOI: 10.33393/jcb.2023.2629
Analytical performance of the FDA-cleared Parsortix® PC1 system
Abstract
Introduction: The Parsortix® PC1 system, Food and Drug Administration (FDA) cleared for use in metastatic breast cancer (MBC) patients, is an epitope-independent microfluidic device for the capture and harvest of circulating tumor cells from whole blood based on cell size and deformability. This report details the analytical characterization of linearity, detection limit, precision, and reproducibility for this device.
Methods: System performance was determined using K2-EDTA blood samples collected from self-declared healthy female volunteers (HVs) and MBC patients spiked with prelabeled cultured breast cancer cell lines (SKBR3, MCF7, or Hs578T). Samples were processed on Parsortix® PC1 systems and captured cells were harvested and enumerated.
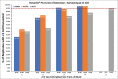
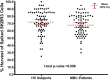
Results: The system captured and harvested live SKBR3, MCF7, and Hs578T cells and fixed SKBR3 cells linearly between 2 and ~100 cells, with average harvest rates of 69%, 73%, 79%, and 90%, respectively. To harvest ≥1 cell ≥95% of the time, the system required 3, 5 or 4 live SKBR3, MCF7 or Hs578T cells, respectively. Average harvest rates from precision studies using 5, 10, and ~50 live cells spiked into blood for each cell line ranged from 63.5% to 76.2%, with repeatability and reproducibility percent coefficient of variation (%CV) estimates ranging from 12.3% to 32.4% and 13.3% to 34.1%, respectively. Average harvest rates using ~20 fixed SKBR3 cells spiked into HV and MBC patient blood samples were 75.0% ± 16.1% (%CV = 22.3%) and 68.4% ± 14.3% (%CV = 21.1%), respectively.
Conclusions: These evaluations demonstrate the Parsortix® PC1 system linearly and reproducibly harvests tumor cells from blood over a range of 1 to ~100 cells.
Keywords: Blood; Breast cancer; Circulating tumor cells; Liquid biopsy; Microfluidic devices; Neoplastic cells.
Copyright © 2023, The Authors.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

