Impact of beta-blockers on mortality and cardiovascular disease outcomes in patients with obstructive sleep apnoea: a population-based cohort study in target trial emulation framework
- PMID: 37601338
- PMCID: PMC10432194
- DOI: 10.1016/j.lanepe.2023.100715
Impact of beta-blockers on mortality and cardiovascular disease outcomes in patients with obstructive sleep apnoea: a population-based cohort study in target trial emulation framework
Abstract
Background: There is no real-world evidence regarding the association between beta-blocker use and mortality or cardiovascular outcomes in patients with obstructive sleep apnoea (OSA). We aimed to investigate the impact of beta-blocker use on all-cause mortality and cardiovascular diseases (CVDs) in patients with OSA.
Methods: We conducted a target trial emulation study of 37,581 patients with newly diagnosed OSA from 1st January 2000 to 30th November 2021 using the IMRD-UK database (formerly known as the THIN database). We compared the treatment strategies of initiating beta-blocker treatment within one year versus non-beta-blocker treatment through the method of clone-censor-weight. Covariates, including patients' demographics, lifestyle, comorbidities, and recent medications, were measured and controlled. Patients were followed up for all-cause mortality or composite CVD outcomes (angina, myocardial infarction, stroke/transient ischaemic attack, heart failure, or atrial fibrillation). We estimated the five-year absolute risks, risk differences and risk ratio with 95% confidence intervals (CIs) with standardised, weighted pooled logistic regression, which is a discrete-time hazard model for survival analysis. Several sensitivity analyses were performed, including multiple imputation addressing the missing data.
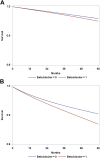
Findings: The median follow-up time was 4.1 (interquartile range, 1.9-7.8) years. The five-year absolute risk of all-cause mortality and CVD outcomes were 4.9% (95% CI, 3.8-6.0) and 13.0% (95% CI, 11.4-15.0) among beta-blocker users, and 4.0% (95% CI, 3.8-4.2) and 9.4% (95% CI, 9.1-9.7) among non-beta-blocker users, respectively. The five-year absolute risk difference and risk ratio between the two groups for all-cause mortality and CVD outcomes were 0.9% (95% CI, -0.2 to 2.1) and 1.22 (95% CI, 0.96-1.54), and 3.5% (95% CI, 2.1-5.5) and 1.37 (95% CI, 1.22-1.62), respectively. Findings were consistent across the sensitivity analyses.
Interpretation: Beta-blocker treatment was associated with an increased risk of CVD and a trend for an increased risk of mortality among patients with OSA. Further studies are needed to confirm our findings.
Funding: Innovation and Technology Commission of the Hong Kong Special Administration Region Government.
Keywords: Beta-blocker; Cohort study; Obstructive sleep apnoea; Trial emulation.
© 2023 The Author(s).
Conflict of interest statement
All authors have completed the ICMJE uniform disclosure form at https://www.thelancet.com/for-authors/forms?section=icmje-coi. ISM reports research grant income to her institution from Menarini, EMA, Sanofi, HDR UK, British Heart Foundation, NIHR HTA and IMI outside the submitted work, institutional consultancy income from AstraZeneca outside the submitted work and personal income from AstraZeneca, Amgen and Amarin outside the submitted work. TMM reports grants from the British Heart Foundation, The National Institute for Health Research Health Technology Assessment (NIHR HTA), Menarini, MSD. Personal income from AstraZeneca (advice on patient/public involvement), Novartis (steering committee), Viatris (lecture fees), Novartis (DSMB), he also served on the HEAT study DSMB (NIHR HTA funded) and he is a trustee of the Scottish Heart Arterial Risk Prevention (SHARP) organisation. KKCM reported receiving grants from the C W Maplethorpe Fellowship, European Union Horizon 2020, National Institute for Health and Care Research (NIHR), and the Innovation and Technology Commission of the Government of the Hong Kong Special Administration Region, and the Hong Kong Research Grants Council (RGC) and receiving personal fees from IQVIA Ltd outside the submitted work. AC, CJ, ADS and LW declare no competing interests.
Figures
References
-
- Foundation TBL . 2015. Obstructive Sleep Apnea (OSA)https://www.blf.org.uk/sites/default/files/OSA_Toolkit_2015_BLF_0.pdf
-
- Pankow W., Nabe B., Lies A., et al. Influence of sleep apnea on 24-hour blood pressure. Chest. 1997;112(5):1253–1258. - PubMed
LinkOut - more resources
Full Text Sources