Integrating proteomic data with metabolic modeling provides insight into key pathways of Bordetella pertussis biofilms
- PMID: 37601354
- PMCID: PMC10435875
- DOI: 10.3389/fmicb.2023.1169870
Integrating proteomic data with metabolic modeling provides insight into key pathways of Bordetella pertussis biofilms
Abstract
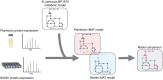
Pertussis, commonly known as whooping cough is a severe respiratory disease caused by the bacterium, Bordetella pertussis. Despite widespread vaccination, pertussis resurgence has been observed globally. The development of the current acellular vaccine (ACV) has been based on planktonic studies. However, recent studies have shown that B. pertussis readily forms biofilms. A better understanding of B. pertussis biofilms is important for developing novel vaccines that can target all aspects of B. pertussis infection. This study compared the proteomic expression of biofilm and planktonic B. pertussis cells to identify key changes between the conditions. Major differences were identified in virulence factors including an upregulation of toxins (adenylate cyclase toxin and dermonecrotic toxin) and downregulation of pertactin and type III secretion system proteins in biofilm cells. To further dissect metabolic pathways that are altered during the biofilm lifestyle, the proteomic data was then incorporated into a genome scale metabolic model using the Integrative Metabolic Analysis Tool (iMAT). The generated models predicted that planktonic cells utilised the glyoxylate shunt while biofilm cells completed the full tricarboxylic acid cycle. Differences in processing aspartate, arginine and alanine were identified as well as unique export of valine out of biofilm cells which may have a role in inter-bacterial communication and regulation. Finally, increased polyhydroxybutyrate accumulation and superoxide dismutase activity in biofilm cells may contribute to increased persistence during infection. Taken together, this study modeled major proteomic and metabolic changes that occur in biofilm cells which helps lay the groundwork for further understanding B. pertussis pathogenesis.
Keywords: Bordetella pertussis; infectious disease; label free quantification (LFQ); mass spectrometry; metabolic model; proteomics; whooping cough.
Copyright © 2023 Suyama, Luu, Zhong, Raftery and Lan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
The vaccine potential of Bordetella pertussis biofilm-derived membrane proteins.Emerg Microbes Infect. 2014 Aug;3(8):e58. doi: 10.1038/emi.2014.58. Epub 2014 Aug 20. Emerg Microbes Infect. 2014. PMID: 26038752 Free PMC article.
-
Shotgun proteomic analysis of Bordetella parapertussis provides insights into the physiological response to iron starvation and potential new virulence determinants absent in Bordetella pertussis.J Proteomics. 2019 Aug 30;206:103448. doi: 10.1016/j.jprot.2019.103448. Epub 2019 Jul 17. J Proteomics. 2019. PMID: 31325608
-
Probing the Genome-Scale Metabolic Landscape of Bordetella pertussis, the Causative Agent of Whooping Cough.Appl Environ Microbiol. 2017 Oct 17;83(21):e01528-17. doi: 10.1128/AEM.01528-17. Print 2017 Nov 1. Appl Environ Microbiol. 2017. PMID: 28842544 Free PMC article.
-
Molecular aspects of Bordetella pertussis pathogenesis.Int Microbiol. 1999 Sep;2(3):137-44. Int Microbiol. 1999. PMID: 10943406 Review.
-
Bordetella pertussis infection: pathogenesis, diagnosis, management, and the role of protective immunity.Eur J Clin Microbiol Infect Dis. 2000 Feb;19(2):77-88. doi: 10.1007/s100960050435. Eur J Clin Microbiol Infect Dis. 2000. PMID: 10746492 Review.
Cited by
-
Comparative metabolic study of planktonic and sessile cells in Salmonella Enteritidis ATCC 13076: Elucidating metabolic pathways driving biofilm formation.PLoS One. 2025 Jan 24;20(1):e0317420. doi: 10.1371/journal.pone.0317420. eCollection 2025. PLoS One. 2025. PMID: 39854347 Free PMC article.
-
Protective Activity and Safety of Experimental Acellular Pertussis Vaccines Based on Antigenic Complexes Isolated from Biofilm and Planktonic Cultures of Bordetella pertussis.Bull Exp Biol Med. 2024 Jul;177(3):349-352. doi: 10.1007/s10517-024-06187-9. Epub 2024 Aug 10. Bull Exp Biol Med. 2024. PMID: 39126548
References
-
- Alvarez Hayes J., Lamberti Y., Surmann K., Schmidt F., Völker U., Rodriguez M. E. (2015). Shotgun proteome analysis of Bordetella pertussis reveals a distinct influence of iron availability on the bacterial metabolism, virulence, and defense response. Proteomics 15, 2258–2266. doi: 10.1002/pmic.201400512, PMID: - DOI - PubMed
-
- Anziani P., Becker J., Mignon C., Arnaud-Barbe N., Courtois V., Izac M., et al. . (2023). Deep longitudinal multi-omics analysis of Bordetella pertussis cultivated in bioreactors highlights medium starvations and transitory metabolisms, associated to vaccine antigen biosynthesis variations and global virulence regulation. Front. Microbiol. 14:1036386. doi: 10.3389/fmicb.2023.1036386, PMID: - DOI - PMC - PubMed
-
- Arnal L., Grunert T., Cattelan N., de Gouw D., Villalba M. I., Serra D. O., et al. . (2015). Bordetella pertussis isolates from Argentinean whooping cough patients display enhanced biofilm formation capacity compared to Tohama I reference strain. Front. Microbiol. 6:1352. doi: 10.3389/fmicb.2015.01352 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

