Synthesis, optical properties and crystal structure of (E, E)-1,3-(3,4:9,10-dibenzododeca-1,11-diene-5,7-diyne-1,12-di-yl)benzene
- PMID: 37601394
- PMCID: PMC10439418
- DOI: 10.1107/S2056989023006187
Synthesis, optical properties and crystal structure of (E, E)-1,3-(3,4:9,10-dibenzododeca-1,11-diene-5,7-diyne-1,12-di-yl)benzene
Abstract
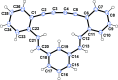
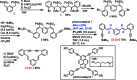
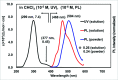
The de-hydro-benzannulene (E,E)-1,3-(3,4:9,10-dibenzododeca-1,11-diene-5,7-diyne-1,12-di-yl)benzene, C26H16, was successfully synthesized via photocatalyst-assisted stereoselective reductive de-sulfonyl-ation of 1,3-bis-{1-phenyl-sulfonyl-2-[2-(tri-methyl-silylethyn-yl)phen-yl]ethen-yl}benzene, C44H42O4S2Si2, and subsequent desilylative cyclization of the resulting (E,E)-bis-silyl-protected dienyne, C32H34Si2. The structure of the de-hydro-benzannulene thus obtained was confirmed by single-crystal X-ray analysis; three benzene rings are connected to one another by a 1,3-butadiynylene and a pair of ethenylene arrays. Although the π-system expanded efficiently in the de-hydro-benzannulene, it was observed that the butadiynylene and ethenylene arrays were strained, showing smaller [171.3 (2)-172.6 (2) °] and larger bond angles [122.5 (2)-131.9 (2)°] than the conventional bond angles, respectively. In CHCl3, the de-hydro-benzannulene showed the longest absorption band at 377 nm. When irradiated by UV light, it emitted fluorescence at 468 nm (ΦF = 0.26) and 504 nm (ΦF = 0.24) in CHCl3 and in the powdered state, respectively.
Keywords: crystal structure; dehydrobenzannulene; expanded π-system; reductive desulfonylation.
© Watanabe et al. 2023.
Figures


Similar articles
-
Synthesis and crystal structure of 5,10-bis-(phenyl-sulfon-yl)tetra-hydro-dibenzo-penta-lene.Acta Crystallogr E Crystallogr Commun. 2025 Jan 28;81(Pt 2):172-176. doi: 10.1107/S205698902500060X. eCollection 2025 Feb 1. Acta Crystallogr E Crystallogr Commun. 2025. PMID: 39927386 Free PMC article.
-
Crystal structures of three 1-oxo-1,2-di-hydro-naphthalene derivatives: dimethyl 4-(4-meth-oxy-phen-yl)-2-(4-methyl-phen-yl)-1-oxo-1,2-di-hydro-naphthalene-2,3-di-carboxyl-ate, dimethyl 1-oxo-2-(pyren-4-yl)-4-(thio-phen-2-yl)-1,2-di-hydro-naphthalene-2,3-di-carboxyl-ate and ethyl 1-oxo-2-phenyl-2,4-bis-(thio-phen-2-yl)-1,2-di-hydro-naphthalene-3-carboxyl-ate.Acta Crystallogr E Crystallogr Commun. 2017 Jan 13;73(Pt 2):177-182. doi: 10.1107/S2056989017000469. eCollection 2017 Feb 1. Acta Crystallogr E Crystallogr Commun. 2017. PMID: 28217337 Free PMC article.
-
Crystal structure of (E)-13-{4-[(Z)-2-cyano-2-(3,4,5-tri-meth-oxy-phen-yl)ethen-yl]phen-yl}parthenolide methanol hemisolvate.Acta Crystallogr Sect E Struct Rep Online. 2014 Sep 6;70(Pt 10):o1092-3. doi: 10.1107/S1600536814019333. eCollection 2014 Oct 1. Acta Crystallogr Sect E Struct Rep Online. 2014. PMID: 25484689 Free PMC article.
-
Crystal structure determination of two pyridine derivatives: 4-[(E)-2-(4-meth-oxy-phen-yl)ethen-yl]-1-methyl-pyridin-1-ium hexa-fluoro-λ6-phosphane and 4-{(E)-2-[4-(di-methyl-amino)-phen-yl]ethen-yl}-1-phenyl-1λ5-pyridin-1-ylium hexa-fluoro-λ6-phosphane.Acta Crystallogr E Crystallogr Commun. 2019 Jan 31;75(Pt 2):288-291. doi: 10.1107/S2056989019001403. eCollection 2019 Feb 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 30800469 Free PMC article.
-
Crystal structures of three indole derivatives: 3-ethnyl-2-methyl-1-phenyl-sulfonyl-1H-indole, 4-phenyl-sulfonyl-3H,4H-cyclo-penta-[b]indol-1(2H)-one and 1-{2-[(E)-2-(5-chloro-2-nitro-phen-yl)ethen-yl]-1-phenyl-sulfonyl-1H-indol-3-yl}ethan-1-one chloro-form monosolvate.Acta Crystallogr E Crystallogr Commun. 2015 Aug 15;71(Pt 9):1036-41. doi: 10.1107/S2056989015014917. eCollection 2015 Sep 1. Acta Crystallogr E Crystallogr Commun. 2015. PMID: 26396842 Free PMC article.
Cited by
-
Synthesis and crystal structure of 5,10-bis-(phenyl-sulfon-yl)tetra-hydro-dibenzo-penta-lene.Acta Crystallogr E Crystallogr Commun. 2025 Jan 28;81(Pt 2):172-176. doi: 10.1107/S205698902500060X. eCollection 2025 Feb 1. Acta Crystallogr E Crystallogr Commun. 2025. PMID: 39927386 Free PMC article.
References
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
-
- Esser, B., Rominger, F. & Gleiter, R. (2008). J. Am. Chem. Soc. 130, 6716–6717. - PubMed
-
- Karki, S., Karas, L. J., Wang, X., Wu, J. I. & Miljanić, O. Š. (2022). Cryst. Growth Des. 22, 2076–2081.
-
- Ojima, J., Kakumi, H., Kitatani, K., Wada, K., Ejiri, E. & Nakada, T. (1985). Can. J. Chem. 63, 2885–2891.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
