Teduglutide in amyloidosis-associated intestinal failure
- PMID: 37601424
- PMCID: PMC10433832
- DOI: 10.1002/ccr3.7653
Teduglutide in amyloidosis-associated intestinal failure
Abstract
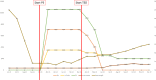
Amyloidosis is a heterogeneous disease characterized by tissue deposition of abnormally folded fibrillary proteins that can manifest itself by a wide variety of symptoms depending on the affected organs. GI involvement among amyloidosis patients is common. Its clinical manifestation often presents with nonspecific symptoms such as weight loss, diarrhea, and malabsorption. With no specific treatment existing for GI amyloidosis, therapy focuses on impeding amyloid deposition and managing the patients' symptoms with supportive measures. Here, we present an AL-amyloidosis patient with GI involvement and intestinal failure (IF) who was successfully treated with the glucagon-like peptide-2 (GLP-2) analogue teduglutide. Over the course of treatment with teduglutide, the patient was able to achieve independence from parenteral nutrition and experienced a significant improvement in quality of life (QoL) as stool frequency and consistency improved, urinary output was stabilized and body weight as well as body composition improved over the course of teduglutide therapy. With no longer being exposed to the burden and associated risks of parenteral nutrition, we were able to reduce the potential morbidity and mortality rate as well as to improve the patient's overall QoL. Intestinal tissue biopsy workup revealed a histopathological correlate for the clinical response; Congo-Red-positive intestinal depositions almost completely disappeared within 6 months of teduglutide therapy. Implementing intestinotrophic GLP-2 analogue teduglutide may enrich the spectrum of treatment options for amyloidosis patients with IF who are dependent on parenteral support.
Keywords: amyloidosis; enteral autonomy; glucagon‐like peptide‐2; intestinal failure; teduglutide.
© 2023 The Authors. Clinical Case Reports published by John Wiley & Sons Ltd.
Conflict of interest statement
Felix Harpain reports a grant and lecture fee from Takeda, outside the submitted work. Elisabeth Hütterer and Anton Stift report lecture fees from Takeda, outside the submitted work. Clara Luhn, Hermine Agis, Ingrid Simonitsch‐Klupp, and Christopher Dawoud have indicated they have no conflict of interests to disclose.
Figures

References
-
- Lachmann HJ, Hawkins PN. Systemic amyloidosis. Curr Opin Pharmacol. 2006;6(2):214‐220. - PubMed
-
- Merlini G. Systemic amyloidosis: are we moving ahead? Neth J Med. 2004;62(4):104‐105. - PubMed
-
- Wechalekar AD, Gillmore JD, Hawkins PN. Systemic amyloidosis. Lancet. 2016;387(10038):2641‐2654. - PubMed
-
- Muchtar E, Dispenzieri A, Magen H, et al. Systemic amyloidosis from a (AA) to T (ATTR): a review. J Intern Med. 2021;289(3):268‐292. - PubMed
-
- Ebert EC, Nagar M. Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol. 2008;103(3):776‐787. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

