A mathematical model to serve as a clinical tool for assessing obstructive sleep apnea severity
- PMID: 37601632
- PMCID: PMC10434550
- DOI: 10.3389/fphys.2023.1198132
A mathematical model to serve as a clinical tool for assessing obstructive sleep apnea severity
Abstract
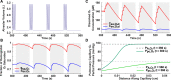
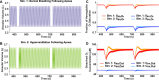
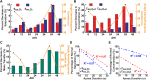
Obstructive sleep apnea (OSA) is a sleep disorder caused by periodic airway obstructions and has been associated with numerous health consequences, which are thought to result from tissue hypoxia. However, challenges in the direct measurement of tissue-level oxygenation make it difficult to analyze the hypoxia exposure pattern in patients. Furthermore, current clinical practice relies on the apnea-hypopnea index (AHI) and pulse oximetry to assess OSA severity, both of which have limitations. To overcome this, we developed a clinically deployable mathematical model, which outputs tissue-level oxygenation. The model incorporates spatial pulmonary oxygen uptake, considers dissolved oxygen, and can use time-dependent patient inputs. It was applied to explore a series of breathing patterns that are clinically differentiated. Supporting previous studies, the result of this analysis indicated that the AHI is an unreliable indicator of hypoxia burden. As a proof of principle, polysomnography data from two patients was analyzed with this model. The model showed greater sensitivity to breathing in comparison with pulse oximetry and provided systemic venous oxygenation, which is absent from clinical measurements. In addition, the dissolved oxygen output was used to calculate hypoxia burden scores for each patient and compared to the clinical assessment, highlighting the importance of event length and cumulative impact of obstructions. Furthermore, an intra-patient statistical analysis was used to underscore the significance of closely occurring obstructive events and to highlight the utility of the model for quantitative data processing. Looking ahead, our model can be used with polysomnography data to predict hypoxic burden on the tissues and help guide patient treatment decisions.
Keywords: breathing; desaturation; hypopnea; hypoxemia; hypoxia; mass transfer; oxygenation.
Copyright © 2023 Qayyum, Wallace, Khayat and Grosberg.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Effects of opioid, hypnotic and sedating medications on sleep-disordered breathing in adults with obstructive sleep apnoea.Cochrane Database Syst Rev. 2015 Jul 14;(7):CD011090. doi: 10.1002/14651858.CD011090.pub2. Cochrane Database Syst Rev. 2015. PMID: 26171909
-
Drug therapy for obstructive sleep apnoea in adults.Cochrane Database Syst Rev. 2013 May 31;2013(5):CD003002. doi: 10.1002/14651858.CD003002.pub3. Cochrane Database Syst Rev. 2013. PMID: 23728641 Free PMC article.
-
Practice parameters for the surgical modifications of the upper airway for obstructive sleep apnea in adults.Sleep. 2010 Oct;33(10):1408-13. doi: 10.1093/sleep/33.10.1408. Sleep. 2010. PMID: 21061864 Free PMC article.
-
Assessing the comparative effects of interventions in COPD: a tutorial on network meta-analysis for clinicians.Respir Res. 2024 Dec 21;25(1):438. doi: 10.1186/s12931-024-03056-x. Respir Res. 2024. PMID: 39709425 Free PMC article. Review.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
Cited by
-
Improving the cardiovascular outcomes of obstructive sleep apnea: Towards more precise hypoxia-based models of disease severity.Curr Sleep Med Rep. 2025;11(1):3. doi: 10.1007/s40675-024-00315-7. Epub 2025 Jan 3. Curr Sleep Med Rep. 2025. PMID: 40416582
-
A Dynamic Fitting Strategy for Physiological Models: A Case Study of a Cardiorespiratory Model for the Simulation of Incremental Aerobic Exercise.J Pers Med. 2024 Apr 11;14(4):406. doi: 10.3390/jpm14040406. J Pers Med. 2024. PMID: 38673033 Free PMC article.
References
-
- Azarbarzin A., Sands S. A., Stone K. L., Taranto-Montemurro L., Messineo L., Terrill P. I., et al. (2019). The hypoxic burden of sleep apnoea predicts cardiovascular disease-related mortality: The osteoporotic fractures in men study and the sleep heart health study. Eur. Heart J. 40, 1149–1157. 10.1093/eurheartj/ehy624 - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

