Randomized double-blind, placebo-controlled multicenter phase III study of prevention of irinotecan-induced diarrhea by a probiotic mixture containing Bifidobacterium BB-12®Lactobacillus rhamnosus LGG® in colorectal cancer patients
- PMID: 37601667
- PMCID: PMC10438450
- DOI: 10.3389/fonc.2023.1168654
Randomized double-blind, placebo-controlled multicenter phase III study of prevention of irinotecan-induced diarrhea by a probiotic mixture containing Bifidobacterium BB-12®Lactobacillus rhamnosus LGG® in colorectal cancer patients
Abstract
Background: The incidence of irinotecan-induced diarrhea varies between 60-90%, by which the incidence of severe diarrhea is 20-40%. The objective of this phase III trial was to determine the effectiveness of the probiotic mixture containing Bifidobacterium, BB-12® and Lactobacillus rhamnosus, LGG® in the prophylaxis of irinotecan-induced diarrhea in metastatic colorectal cancer patients due to a reduction in the activity of intestinal beta-D-glucuronidase.
Methods: From March 2016 to May 2022, a total of 242 patients with colorectal cancer starting a new line of irinotecan-based therapy were registered to the study in 11 cancer centers in Slovakia. Patients were randomized in a ratio 1:1 to probiotic formula vs. placebo that was administered for 6 weeks. Each capsule of Probio-Tec® BG-Vcap-6.5 contained 2.7x109 colony-forming units (CFU) of 2 lyophilized probiotic strains Bifidobacterium, BB-12® (50%) and Lactobacillus rhamnosus GG, LGG® (50%).
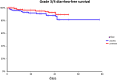
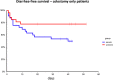
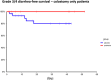
Results: Administration of probiotics compared to placebo was not associated with a significant reduction of grade 3/4 diarrhea (placebo arm 11.8% vs. probiotic arm 7.9%, p=0.38). Neither the overall incidence of diarrhea (46.2% vs. 41.2%, p=0.51) nor the incidence of enterocolitis (3.4% vs. 0.9%, p=0.37) was different in the placebo vs. probiotic arm. Subgroup analysis revealed that patients with colostomy had higher incidence of any diarrhea and grade 3/4 diarrhea in the placebo arm compared to the probiotic arm (48.5% vs. 22.2%, p=0.06 and 15.2% vs. 0%, p=0.06, respectively). Moreover, patients on probiotic arm had significantly better diarrhea-free survival (HR = 0.41, 95%CI 0.18 - 0.95, p=0.05) and needed less loperamide (p=0.01) compared to patients on placebo arm. We did not observe any infection caused by probiotic strains used in this study.
Conclusion: This study failed to achieve its primary endpoint, and results suggest a lack of benefit of administered probiotic formula for the prevention of irinotecan-induced diarrhea. However, subgroup analysis suggests a possible benefit in patients with colostomy.
Keywords: beta-glucuronidase; colorectal cancer; diarrhea; irinotecan; probiotics.
Copyright © 2023 Mego, Danis, Chovanec, Jurisova, Bystricky, Porsok, Konkolovsky, Vaclav, Wagnerova, Streško, Brezinova, Rečková, Sutekova, Pazderova, Novisedlakova, Zomborska, Ciernikova, Svetlovska and Drgona.
Conflict of interest statement
Author RD was employed by the company S&D Pharma. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Danis R, Mego M, Antonova M, Stepanova R, Svobodnik A, Hejnova R, et al. . Orally administered probiotics in the prevention of chemotherapy (+/- radiotherapy)-induced gastrointestinal toxicity: A systematic review with meta-analysis of randomized trials. Integr Cancer Ther (2022) 21:15347354221144309. doi: 10.1177/15347354221144309 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

