Ribavirin inhibits the replication of infectious bursal disease virus predominantly through depletion of cellular guanosine pool
- PMID: 37601760
- PMCID: PMC10433155
- DOI: 10.3389/fvets.2023.1192583
Ribavirin inhibits the replication of infectious bursal disease virus predominantly through depletion of cellular guanosine pool
Abstract
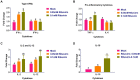
Introduction: The antiviral activity of different mutagens against single-stranded RNA viruses is well documented; however, their activity on the replication of double-stranded RNA viruses remains unexplored. This study aims to investigate the effect of different antivirals on the replication of a chicken embryo fibroblast-adapted Infectious Bursal Disease virus, FVSKG2. This study further explores the antiviral mechanism utilized by the most effective anti-IBDV agent.
Methods: The cytotoxicity and anti-FVSKG2 activity of different antiviral agents (ribavirin, 5-fluorouracil, 5-azacytidine, and amiloride) were evaluated. The virus was serially passaged in chicken embryo fibroblasts 11 times at sub-cytotoxic concentrations of ribavirin, 5-fluorouracil or amiloride. Further, the possible mutagenic and non-mutagenic mechanisms utilized by the most effective anti-FVSKG2 agent were explored.
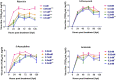
Results and discussion: Ribavirin was the least cytotoxic on chicken embryo fibroblasts, followed by 5-fluorouracil, amiloride and 5-azacytidine. Ribavirin inhibited the replication of FVSKG2 in chicken embryo fibroblasts significantly at concentrations as low as 0.05 mM. The extinction of FVSKG2 was achieved during serial passage of the virus in chicken embryo fibroblasts at ≥0.05 mM ribavirin; however, the emergence of a mutagen-resistant virus was not observed until the eleventh passage. Further, no mutation was observed in 1,898 nucleotides of the FVSKG2 following its five passages in chicken embryo fibroblasts in the presence of 0.025 mM ribavirin. Ribavarin inhibited the FVSKG2 replication in chicken embryo fibroblasts primarily through IMPDH-mediated depletion of the Guanosine Triphosphate pool of cells. However, other mechanisms like ribavirin-mediated cytokine induction or possible inhibition of viral RNA-dependent RNA polymerase through its interaction with the enzyme's active sites enhance the anti-IBDV effect. Ribavirin inhibits ds- RNA viruses, likely through IMPDH inhibition and not mutagenesis. The inhibitory effect may, however, be augmented by other non-mutagenic mechanisms, like induction of antiviral cytokines in chicken embryo fibroblasts or interaction of ribavirin with the active sites of RNA-dependent RNA polymerase of the virus.
Keywords: antiviral; dsRNA; infectious bursal disease virus; mutagen; ribavirin.
Copyright © 2023 Akram, Gul, Parveez Zia, Hassan, Khatun, Shah, Ahmad, Ganai, Chikan, Kim and Shabir.
Conflict of interest statement
NC is employed by Daskdan Innovations Pvt. Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
LinkOut - more resources
Full Text Sources

