Classification system for nanotechnology-enabled health products with both scientific and regulatory application
- PMID: 37601794
- PMCID: PMC10433195
- DOI: 10.3389/fmed.2023.1212949
Classification system for nanotechnology-enabled health products with both scientific and regulatory application
Abstract
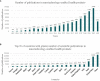
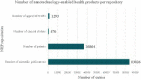
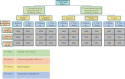
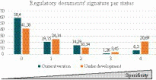
The lack of specific regulatory guidelines for nanotechnology-enabled health products (NHPs) is hampering development and patient access to these innovative technologies. Namely, there is an urgent need for harmonized regulatory definitions and classification systems that allow establishing a standardized framework for NHPs regulatory assessment. In this work, a novel classification system for NHPs is proposed. This classification can be applied for sorting nano-based innovations and regulatory guidelines according to the type of NHPs they address. Said methodology combines scientific and regulatory principles and it is based on the following criteria: principal mode of action, chemical composition, medical purpose and nanomanufacturing approach. This classification system could serve as a useful tool to sensor the state of the art of NHPs which is particularly useful for regulators to support strategy development of regulatory guidelines. Additionally, this tool would also allow manufacturers of NHPs to align their development plans with their applicable guidelines and standards and thus fulfill regulators expectations.
Keywords: classification; nanomedicines; nanotechnology enabled health products; regulatory science; regulatory uncertainties.
Copyright © 2023 Rodríguez-Gómez, Penon, Monferrer and Rivera-Gil.
Conflict of interest statement
Authors FDRG, OP, and DM were employed by company Asphalion SL. The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Halamoda-Kenzaoui B, van Elk M, Gaitan S, Geertsma RE, Lafuente EG, Owen A, et al. . Anticipation of regulatory needs for nanotechnology-enabled health product. Luxembourg: European Commission; (2019) Report No.: JRC118190.
-
- Nourizadeh H, Doostkam M, Seyfi M, Nori M, Mirzaei H, Abbasnia M, et al. . Nano-pharmaceutical products. (2021).
LinkOut - more resources
Full Text Sources

