A comparison of peripheral blood stem cell collection outcomes for multiple myeloma; mobilization matters in the era of IMiD induction
- PMID: 37601867
- PMCID: PMC10435720
- DOI: 10.1002/jha2.702
A comparison of peripheral blood stem cell collection outcomes for multiple myeloma; mobilization matters in the era of IMiD induction
Abstract
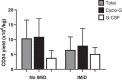
Collection of peripheral blood stem cells (PBSCs) for autologous stem cell transplant (ASCT) requires mobilization from the bone marrow. There is variation in mobilization choice; during the COVID-19 pandemic BSBMT&CT guidelines recommended using granulocyte-colony stimulating factor (G-CSF) alone to minimize the use of chemotherapy. We report on the impact of mobilization regimen on stem cell collection, and whether IMiD-containing induction therapy impacts on mobilization and consequently transplant engraftment times for 83 patients undergoing ASCT at Leeds Teaching Hospitals. Cyclophosphamide plus G-CSF (cyclo-G) mobilization yielded more CD34+ cells (8.94 vs. 4.88 ×106/kg, p = < 0.0001) over fewer days (1.6 vs. 2.4 days, p = 0.007), and required fewer doses of salvage Plerixafor than G-CSF only (13.6% vs. 35%, p = 0.0407). IMiD-containing induction impaired all of these factors. CD34+ doses > 8×106/kg were more frequent with Cyclo-G (62% vs. 11%, p = 0.0001), including for those receiving IMiD 1st line induction (50% vs. 13.3%, p = 0.0381). Note that 92.6% of those receiving IMiD-free inductions were mobilized with Cyclo-G. The novel agents used in modern induction regimens (e.g Daratumumab) have been shown to impair yields, increasing the importance of optimizing mobilization regimens in the first instance. Furthermore, as cellular therapies become established in the management of multiple myeloma emerging data highlights the potential benefits of stem cell top up in the management of the haematological toxicities of these therapies. Our findings support re-adoption of Cyclo-G as the gold standard for mobilization to optimize PBSC harvesting and ensure sufficient cells for subsequent ASCTs.
Keywords: myeloma; stem cell mobilization/homing; stem cell transplantation.
© 2023 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
The authors have no competing interests to declare
Figures
Similar articles
-
Stem Cell Mobilization Yields with Daratumumab- and Lenalidomide-Containing Quadruplet Induction Therapy in Newly Diagnosed Multiple Myeloma: Findings from the MASTER and GRIFFIN Trials.Transplant Cell Ther. 2023 Mar;29(3):174.e1-174.e10. doi: 10.1016/j.jtct.2022.11.029. Epub 2022 Dec 6. Transplant Cell Ther. 2023. PMID: 36494017
-
Stem Cell Mobilization for Multiple Myeloma Patients Receiving Daratumumab-Based Induction Therapy: A Real- World Experience.Transplant Cell Ther. 2023 May;29(5):340.e1-340.e4. doi: 10.1016/j.jtct.2023.02.013. Epub 2023 Feb 19. Transplant Cell Ther. 2023. PMID: 36804934
-
A comparison of chemo-free strategy with G-CSF plus plerixafor on demand versus intermediate-dose cyclophosphamide and G-CSF as PBSC mobilization in newly diagnosed multiple myeloma patients: An Italian explorative cost Analysis.Transfus Apher Sci. 2020 Oct;59(5):102819. doi: 10.1016/j.transci.2020.102819. Epub 2020 May 25. Transfus Apher Sci. 2020. PMID: 32499108
-
Plerixafor: A chemokine receptor-4 antagonist for mobilization of hematopoietic stem cells for transplantation after high-dose chemotherapy for non-Hodgkin's lymphoma or multiple myeloma.Clin Ther. 2010 May;32(5):821-43. doi: 10.1016/j.clinthera.2010.05.007. Clin Ther. 2010. PMID: 20685493 Review.
-
Plerixafor: a review of its use in stem-cell mobilization in patients with lymphoma or multiple myeloma.Drugs. 2011 Aug 20;71(12):1623-47. doi: 10.2165/11206040-000000000-00000. Drugs. 2011. PMID: 21861545 Review.
Cited by
-
Impact of Anti-CD38 Monoclonal Antibody Therapy on CD34+ Hematopoietic Stem Cell Mobilization, Collection, and Engraftment in Multiple Myeloma Patients-A Systematic Review.Pharmaceuticals (Basel). 2024 Jul 15;17(7):944. doi: 10.3390/ph17070944. Pharmaceuticals (Basel). 2024. PMID: 39065794 Free PMC article. Review.
References
-
- Karakantza M. Datakindly provided from NHS Blood and Transplantregistry 2020. (Unpublished)
-
- BSBMTCT COVID‐19 Vaccination Sub‐Group . BSBMT&CT recommendations for the management of adult patients and allogeneic donors during the COVID‐19 (causative agent the SARS‐CoV‐2 virus) outbreak. [Internet] Version 5; 4th December 2020 (clinical guideline). Accessed 22nd January 2023. Available from: https://bsbmtct.org/wp‐content/uploads/2020/12/BSBMTCT‐COVID‐19‐Guidelin...
-
- Wang L, Xiang H, Yan Y, Deng Z, Li H, Li X, et al. Comparison of the efficiency, safety, and survival outcomes in two stem cell mobilization regimens with cyclophosphamide plus G‐CSF or G‐CSF alone in multiple myeloma: a meta‐analysis. Ann Hematol. 2021;100(2):563–73. 10.1007/s00277-020-04376-w. PMID: 33404694; PMCID: PMC7817584 - DOI - PMC - PubMed
-
- Arora M, Burns LJ, Barker JN, Miller JS, Defor TE, Olujohungbe AB, et al. Randomized comparison of granulocyte colony‐stimulating factor versus granulocyte‐macrophage colony stimulating factor plus intensive chemotherapy for peripheral blood stem cell mobilization and autologous transplantation in multiple myeloma. Biol Blood Marrow Transplant. 2004;10(6):395–404. - PubMed
-
- Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem‐cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open‐label, phase 3 study. Lancet. 2019;394(10192):29–38. 10.1016/S0140-6736(19)31240-1. PMID: 31171419. - DOI - PubMed
LinkOut - more resources
Full Text Sources