Normalisation of haemoglobin and control of breakthrough haemolysis with increased frequency pegcetacoplan dosing in treated paroxysmal nocturnal haemoglobinuria
- PMID: 37601872
- PMCID: PMC10435701
- DOI: 10.1002/jha2.714
Normalisation of haemoglobin and control of breakthrough haemolysis with increased frequency pegcetacoplan dosing in treated paroxysmal nocturnal haemoglobinuria
Abstract
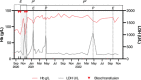
Paroxysmal nocturnal haemoglobinura is an acquired life-threatening haemolytic condition, which is generally well controlled with terminal complement blockade with eculizumab. Whilst almost all patients treated with terminal complement inhibitors develop extravascular haemolysis, only a small proportion of these results in symptomatic anaemia limiting their activities and requiring red cell transfusion. This case highlights the potential role for the C3 inhibitor, pegcetacoplan, in controlling both intravascular and extravascular haemolysis, and is the first case to report on the use of additional doses of pegcetacoplan to control breakthrough haemolysis.
Keywords: PNH; breakthrough haemolysis; pegcetacoplan.
© 2023 The Authors. eJHaem published by British Society for Haematology and John Wiley & Sons Ltd.
Conflict of interest statement
AKD: advisory board membership for Sobi. JS: consultancies, advisory board membership and speaker for Alexion, Apellis, Sobi, Novartis, Takeda, Pfizer. NB has nothing to declare.
Figures
Similar articles
-
Pegcetacoplan: A Review in Paroxysmal Nocturnal Haemoglobinuria.Drugs. 2022 Dec;82(18):1727-1735. doi: 10.1007/s40265-022-01809-w. Epub 2022 Dec 2. Drugs. 2022. PMID: 36459381 Free PMC article. Review.
-
Pegcetacoplan versus eculizumab in patients with paroxysmal nocturnal haemoglobinuria (PEGASUS): 48-week follow-up of a randomised, open-label, phase 3, active-comparator, controlled trial.Lancet Haematol. 2022 Sep;9(9):e648-e659. doi: 10.1016/S2352-3026(22)00210-1. Lancet Haematol. 2022. PMID: 36055332 Clinical Trial.
-
Pegcetacoplan: First Approval.Drugs. 2021 Aug;81(12):1423-1430. doi: 10.1007/s40265-021-01560-8. Drugs. 2021. PMID: 34342834 Review.
-
Addition of iptacopan, an oral factor B inhibitor, to eculizumab in patients with paroxysmal nocturnal haemoglobinuria and active haemolysis: an open-label, single-arm, phase 2, proof-of-concept trial.Lancet Haematol. 2021 May;8(5):e344-e354. doi: 10.1016/S2352-3026(21)00028-4. Epub 2021 Mar 23. Lancet Haematol. 2021. PMID: 33765419 Clinical Trial.
-
Novel targeted C3 inhibitor pegcetacoplan for paroxysmal nocturnal hemoglobinuria.Clin Exp Med. 2023 Jul;23(3):717-726. doi: 10.1007/s10238-022-00830-3. Epub 2022 Apr 19. Clin Exp Med. 2023. PMID: 35441351 Review.
Cited by
-
Three Years On: The Role of Pegcetacoplan in Paroxysmal Nocturnal Hemoglobinuria (PNH) since Its Initial Approval.Int J Mol Sci. 2024 Aug 9;25(16):8698. doi: 10.3390/ijms25168698. Int J Mol Sci. 2024. PMID: 39201383 Free PMC article. Review.
References
-
- Hillmen P, Young NS, Schubert J, Brodsky RA, Socie G, Muus P, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–43. - PubMed
-
- Brodsky RA, Young NS, Antonioli E, Risitano AM, Schrezenmeier H, Schubert J, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood.2008;111(4):1840–7. - PubMed
-
- Kelly RJ, Hill A, Arnold LM, Brooksbank GL, Richards SJ, Cullen M, et al. Long‐term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117(25):6786–92. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
