A Quantitative Description for Optical Mass Measurement of Single Biomolecules
- PMID: 37602293
- PMCID: PMC10436351
- DOI: 10.1021/acsphotonics.3c00422
A Quantitative Description for Optical Mass Measurement of Single Biomolecules
Abstract
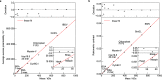
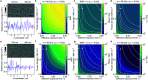
Label-free detection of single biomolecules in solution has been achieved using a variety of experimental approaches over the past decade. Yet, our understanding of the magnitude of the optical contrast and its relationship with the underlying atomic structure as well as the achievable measurement sensitivity and precision remain poorly defined. Here, we use a Fourier optics approach combined with an atomic structure-based molecular polarizability model to simulate mass photometry experiments from first principles. We find excellent agreement between several key experimentally determined parameters such as optical contrast-to-mass conversion, achievable mass accuracy, and molecular shape and orientation dependence. This allows us to determine detection sensitivity and measurement precision mostly independent of the optical detection approach chosen, resulting in a general framework for light-based single-molecule detection and quantification.
© 2023 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): P.K. is a nonexecutive director and shareholder of Refeyn Ltd., while I.C. is an employee of Refeyn Ltd. (his work has been carried out while being a student at University of Oxford). The other authors declare no competing interests.
Figures



References
-
- Young G.; Hundt N.; Cole D.; Fineberg A.; Andrecka J.; Tyler A.; Olerinyova A.; Ansari A.; Marklund E. G.; Collier M. P.; Chandler S. A.; Tkachenko O.; Allen J.; Crispin M.; Billington N.; Takagi Y.; Sellers J. R.; Eichmann C.; Selenko P.; Frey L.; Riek R.; Galpin M. R.; Struwe W. B.; Benesch J. L. P.; Kukura P. Quantitative Mass Imaging of Single Biological Macromolecules. Science 2018, 360, 423–427. 10.1126/science.aar5839. - DOI - PMC - PubMed
-
- Špačková B.; Klein Moberg H.; Fritzsche J.; Tenghamn J.; Sjösten G.; Šípová-Jungová H.; Albinsson D.; Lubart Q.; van Leeuwen D.; Westerlund F.; Midtvedt D.; Esbjörner E. K.; Käll M.; Volpe G.; Langhammer C. Label-Free Nanofluidic Scattering Microscopy of Size and Mass of Single Diffusing Molecules and Nanoparticles. Nat. Methods 2022, 19, 751–758. 10.1038/s41592-022-01491-6. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
