The role of p53 in anti-tumor immunity and response to immunotherapy
- PMID: 37602328
- PMCID: PMC10434531
- DOI: 10.3389/fmolb.2023.1148389
The role of p53 in anti-tumor immunity and response to immunotherapy
Abstract
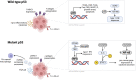
p53 is a transcription factor that regulates the expression of genes involved in tumor suppression. p53 mutations mediate tumorigenesis and occur in approximately 50% of human cancers. p53 regulates hundreds of target genes that induce various cell fates including apoptosis, cell cycle arrest, and DNA damage repair. p53 also plays an important role in anti-tumor immunity by regulating TRAIL, DR5, TLRs, Fas, PKR, ULBP1/2, and CCL2; T-cell inhibitory ligand PD-L1; pro-inflammatory cytokines; immune cell activation state; and antigen presentation. Genetic alteration of p53 can contribute to immune evasion by influencing immune cell recruitment to the tumor, cytokine secretion in the TME, and inflammatory signaling pathways. In some contexts, p53 mutations increase neoantigen load which improves response to immune checkpoint inhibition. Therapeutic restoration of mutated p53 can restore anti-cancer immune cell infiltration and ameliorate pro-tumor signaling to induce tumor regression. Indeed, there is clinical evidence to suggest that restoring p53 can induce an anti-cancer immune response in immunologically cold tumors. Clinical trials investigating the combination of p53-restoring compounds or p53-based vaccines with immunotherapy have demonstrated anti-tumor immune activation and tumor regression with heterogeneity across cancer type. In this Review, we discuss the impact of wild-type and mutant p53 on the anti-tumor immune response, outline clinical progress as far as activating p53 to induce an immune response across a variety of cancer types, and highlight open questions limiting effective clinical translation.
Keywords: DNA damage; anti-cancer immunity; immunotherapy; p53; tumor microenvironment.
Copyright © 2023 Carlsen, Zhang, Tian, De La Cruz, George, Arnoff and El-Deiry.
Conflict of interest statement
WSE-D is a co-founder of Oncoceutics, Inc., a subsidiary of Chimerix. WSE-D has disclosed his relationship with Oncoceutics/Chimerix and potential conflict of interest to his academic institution/employer and is fully compliant with NIH and institutional policy that is managing this potential conflict of interest. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Barnes B. J., Kellum M. J., Pinder K. E., Frisancho J. A., Pitha P. M. (2003). Interferon regulatory factor 5, a novel mediator of cell cycle arrest and cell death. Cancer Res. 63 (19), 6424–6431. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

