Addition of long-acting beta2 agonists or long-acting muscarinic antagonists versus doubling the dose of inhaled corticosteroids (ICS) in adolescents and adults with uncontrolled asthma with medium dose ICS: a systematic review and network meta-analysis
- PMID: 37602534
- PMCID: PMC10441001
- DOI: 10.1002/14651858.CD013797.pub2
Addition of long-acting beta2 agonists or long-acting muscarinic antagonists versus doubling the dose of inhaled corticosteroids (ICS) in adolescents and adults with uncontrolled asthma with medium dose ICS: a systematic review and network meta-analysis
Abstract
Background: Inhaled corticosteroids (ICS) are the mainstay treatment for persistent asthma. Escalating treatment is required when asthma is not controlled with ICS therapy alone, which would include, but is not limited to, adding a long-acting beta2-agonist (LABA) or a long-acting muscarinic antagonist (LAMA) or doubling the dose of ICS.
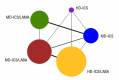
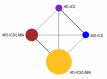
Objectives: To assess the efficacy and safety of adding a LABA or LAMA to ICS therapy versus doubling the dose of ICS in adolescents and adults whose asthma is not well controlled on medium-dose (MD)-ICS using a network meta-analysis (NMA), and to provide a ranking of these treatments according to their efficacy and safety.
Search methods: We searched the Cochrane Airways Trials Register, CENTRAL, MEDLINE, Embase, Global Health, ClinicalTrials.gov, and the World Health Organization ICTRP for pre-registered randomised controlled trials (RCTs) from January 2008 to 19 December 2022.
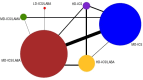
Selection criteria: We searched for studies including adolescents and adults with uncontrolled asthma who had been treated with or were eligible for MD-ICS, comparing it to high-dose (HD)-ICS, ICS/LAMA, or ICS/LABA. We excluded cluster- and cross-over RCTs. Studies were of at least 12 weeks duration.
Data collection and analysis: We conducted a systematic review and network meta-analysis according to a previously published protocol. We used Cochrane's Screen4ME workflow to assess search results. We used Grading of Recommendations Assessment, Development and Evaluation (GRADE) to assess the certainty of evidence. The primary outcome is asthma exacerbations (moderate and severe).
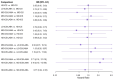
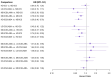
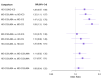
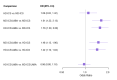
Main results: We included 38,276 participants from 35 studies (median duration 24 weeks (range 12 to 78); mean age 44.1; 38% male; 69% white; mean forced expiratory volume in one second 2.1 litres and 68% of predicted). MD- and HD-ICS/LABA likely reduce and MD-ICS/LAMA possibly reduces moderate to severe asthma exacerbations compared to MD-ICS (hazard ratio (HR) 0.70, 95% credible interval (CrI) 0.59 to 0.82; moderate certainty; HR 0.59, 95% CrI 0.46 to 0.76; moderate certainty; and HR 0.56, 95% CrI 0.38 to 0.82; low certainty, respectively), whereas HD-ICS probably does not (HR 0.94, 95% CrI 0.70 to 1.24; moderate certainty). There is no clear evidence to suggest that any combination therapy or HD-ICS reduces severe asthma exacerbations compared to MD-ICS (low to moderate certainty). This study suggests no clinically meaningful differences in the symptom or quality of life score between dual combinations and monotherapy (low to high certainty). MD- and HD-ICS/LABA increase or likely increase the odds of Asthma Control Questionnaire (ACQ) responders at 6 and 12 months compared to MD-ICS (odds ratio (OR) 1.47, 95% CrI 1.23 to 1.76; high certainty; and OR 1.59, 95% CrI 1.31 to 1.94; high certainty at 6 months; and OR 1.61, 95% CrI 1.22 to 2.13; moderate certainty and OR 1.55, 95% CrI 1.20 to 2.00; high certainty at 12 months, respectively). MD-ICS/LAMA probably increases the odds of ACQ responders at 6 months (OR 1.32, 95% CrI 1.11 to 1.57; moderate certainty). No data were available at 12 months. There is no clear evidence to suggest that HD-ICS increases the odds of ACQ responders or improves the symptom or qualify of life score compared to MD-ICS (very low to high certainty). There is no evidence to suggest that ICS/LABA or ICS/LAMA reduces asthma-related or all-cause serious adverse events (SAEs) compared to MD-ICS (very low to high certainty). HD-ICS results in or likely results in little or no difference in the included safety outcomes compared to MD-ICS as well as HD-ICS/LABA compared to MD-ICS/LABA. The pairwise meta-analysis shows that MD-ICS/LAMA likely reduces all-cause adverse events (AEs) and results in a slight reduction in treatment discontinuation due to AEs compared to MD-ICS (risk ratio (RR) 0.86, 95% confidence interval (CI) 0.77 to 0.96; 4 studies, 2238 participants; moderate certainty; and RR 0.51, 95% CI 0.26 to 0.99; 4 studies, 2239 participants; absolute risk reduction 10 fewer per 1000 participants; moderate certainty, respectively). The NMA evidence is in agreement with the pairwise evidence on treatment discontinuation due to AEs, but very uncertain on all-cause AEs, due to imprecision and heterogeneity.
Authors' conclusions: The review findings suggest that MD- or HD-ICS/LABA and MD-ICS/LAMA reduce moderate to severe asthma exacerbations and increase the odds of ACQ responders compared to MD-ICS whereas HD-ICS probably does not. The evidence is generally stronger for MD- and HD-ICS/LABA than for MD-ICS/LAMA primarily due to a larger evidence base. There is no evidence to suggest that ICS/LABA, ICS/LAMA, or HD-ICS/LABA reduces severe asthma exacerbations or SAEs compared to MD-ICS. MD-ICS/LAMA likely reduces all-cause AEs and results in a slight reduction in treatment discontinuation due to AEs compared to MD-ICS. The above findings may assist in deciding on a treatment option during the stepwise approach of asthma management. Longer-term safety of higher than medium-dose ICS needs to be addressed in phase 4 or observational studies given that the median duration of included studies was six months.
Trial registration: ClinicalTrials.gov NCT00565266.
Copyright © 2023 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Y. Oba has provided consultation and received honoraria from Genentech unrelated to the current review. This author, who is a Cochrane Editor, was not involved in the editorial process.
T Patel: none known.
S Anwer: none known.
T Maduke: none known.
S Dias: none known.
Figures



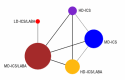





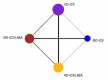

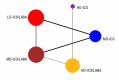


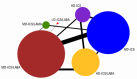



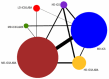
































Update of
- doi: 10.1002/14651858.CD013797
References
References to studies included in this review
Bateman 2014 {published and unpublished data}
Beasley 2015 {published data only}
-
- Beasley RW, Donohue JF, Mehta R, Nelson HS, Clay M, Moton A, et al. Effect of once-daily indacaterol maleate/mometasone furoate on exacerbation risk in adolescent and adult asthma: a double-blind randomised controlled trial. BMJ Open 2015;5(2):e006131. [DOI: 10.1136/bmjopen-2014-006131] [PMID: ] - DOI - PMC - PubMed
Bernstein 2011 {published and unpublished data}
-
- Bernstein DI, Hébert J, Cheema A, Murphy KR, Chérrez-Ojeda I, Matiz-Bueno CE, et al. Efficacy and onset of action of mometasone furoate/formoterol and fluticasone propionate/salmeterol combination treatment in subjects with persistent asthma. Allergy, Asthma & Clinical Immunology 2011;7(1):21. [DOI: ] [PMID: ] - PMC - PubMed
Bernstein 2015 {published data only}
Bernstein 2017 {published data only}
-
- Bernstein DI, Gillespie M, Song S, Steinfeld J. Safety, efficacy, and dose response of fluticasone propionate delivered via the novel MDPI in patients with severe asthma: a randomized, controlled, dose-ranging study. Journal of Asthma 2017;54(6):559-69. [DOI: 10.1080/02770903.2016.1242137] [PMID: ] - DOI - PubMed
Bleecker 2014 {published data only}
-
- Bleecker ER, Lötvall J, O'Byrne PM, Woodcock A, Busse WW, Kerwin EM, et al. Fluticasone furoate-vilanterol 100-25 mcg compared with fluticasone furoate 100 mcg in asthma: a randomized trial. Journal of Allergy and Clinical Immunology: In Practice 2014;2(5):553-61. [PMID: ] - PubMed
Bodzenta‐Lukaszyk 2012 {published data only}
-
- Bodzenta-Lukaszyk A, Buhl R, Balint B, Lomax M, Spooner K, Dissanayake S. Fluticasone/formoterol combination therapy versus budesonide/formoterol for the treatment of asthma: a randomized, controlled, non-inferiority trial of efficacy and safety. Journal of Asthma 2012;49(10):1060-70. [DOI: 10.3109/02770903.2012.719253] [PMID: ] - DOI - PubMed
Brown 2012 {published data only}
-
- Brown RW, O'Brien CD, Martin UJ, Uryniak T, Lampl KL. Long-term safety and asthma control measures with a budesonide/formoterol pressurized metered-dose inhaler in African American asthmatic patients: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2012;130(2):362-7. [DOI: 10.1016/j.jaci.2012.03.028] [PMID: ] - DOI - PubMed
Busse 2008 {published data only}
-
- Busse WW, Shah SR, Somerville L, Parasuraman B, Martin P, Goldman M. Comparison of adjustable- and fixed-dose budesonide/formoterol pressurized metered-dose inhaler and fixed-dose fluticasone propionate/salmeterol dry powder inhaler in asthma patients. Journal of Allergy and Clinical Immunology 2008;121(6):1407-14. [DOI: 10.1016/j.jaci.2008.03.019] [PMID: ] - DOI - PubMed
CHIESI 2009 {unpublished data only}
-
- NCT00862394. A 12-week, multinational, randomised, double blind, double dummy, 4-arm parallel-group study comparing the efficacy and safety of CHF 1535 (fixed combination of beclomethasone dipropionate + formoterol fumarate) 100 + 6 g/actuation inhalation powder, administered via the NEXT™ inhaler, versusCHF 1535 (fixed combination of beclomethasone dipropionate +formoterol fumarate) 100 + 6 g/actuation, via HFA pressurised inhalation solution, in moderate to severe symptomatic asthmatic patients aged 12 years under treatment with inhaled corticosteroids. https://www.clinicaltrialsregister.eu/ctr-search/trial/2008-000401-11/re... (accessed 15 July 2017). [CLINICALTRIALS.GOV: NCT00862394] [EUDRACT NUMBER: 2008-000401-11]
Corren 2013 {published data only}
Cukier 2013 {published data only}
-
- Cukier A, Jacob CM, Rosario Filho NA, Fiterman J, Vianna EO, Hetzel JL, et al. Fluticasone/formoterol dry powder versus budesonide/formoterol in adults and adolescents with uncontrolled or partly controlled asthma. Respiratory Medicine 2013;107(9):1330-8. [DOI: 10.1016/j.rmed.2013.06.018] [PMID: ] - DOI - PubMed
Hamelmann 2016 {published data only}
-
- Hamelmann E, Bateman ED, Vogelberg C, Szefler SJ, Vandewalker M, Moroni-Zentgraf P, et al. Tiotropium add-on therapy in adolescents with moderate asthma: a 1-year randomized controlled trial. Journal of Allergy and Clinical Immunology 2016;138(2):441-50. [DOI: 10.1016/j.jaci.2016.01.011] [PMID: ] - DOI - PubMed
Huchon 2009 {published data only}
Katial 2011 {published data only}
Kerstjens 2015 {published data only}
-
- Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respiratory Medicine 2015;3(5):367-76. [DOI: 10.1016/S2213-2600(15)00031-4] [PMID: ] - DOI - PubMed
Kerstjens 2015a {published data only}
-
- Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respiratory Medicine 2015;3(5):367-76. [DOI: 10.1016/S2213-2600(15)00031-4] [PMID: ] - DOI - PubMed
Kerstjens 2015b {published data only}
-
- Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respiratory Medicine 2015;3(5):367-76. [DOI: 10.1016/S2213-2600(15)00031-4] [PMID: ] - DOI - PubMed
Kerstjens 2020 {published data only}
-
- Kerstjens HAM, Maspero J, Chapman KR, Zyl-Smit RN, Hosoe M, Tanase AM, et al. Once-daily, single-inhaler mometasone-indacaterol-glycopyrronium versus mometasone-indacaterol or twice-daily fluticasone-salmeterol in patients with inadequately controlled asthma (IRIDIUM): a randomised, double-blind, controlled phase 3 study. Lancet Respiratory Medicine 2020;8(10):1000-12. [DOI: 10.1016/S2213-2600(20)30190-9] [PMID: ] - DOI - PubMed
Kerwin 2011 {published and unpublished data}
-
- Kerwin E, Prazma CM, Sutton L, Stempel DA. Safety and efficacy of long-term treatment with fluticasone propionate and salmeterol via DISKUS versus fluticasone propionate alone. Clinical Research and Regulatory Affairs 2011;28(1):14-21. [DOI: 10.3109/10601333.2010.544315] - DOI
Kerwin 2020 {published data only}
-
- Kerwin E, Pascoe S, Bailes Z, Nathan R, Bernstein D, Dahl R, et al. A phase IIb, randomised, parallel-group study: the efficacy, safety and tolerability of once-daily umeclidinium in patients with asthma receiving inhaled corticosteroids. Respiratory Research 2020;21(1):148. [DOI: 10.1186/s12931-020-01400-5] [PMID: ] - DOI - PMC - PubMed
Lee 2020 {published data only}
-
- Lee LA, Bailes Z, Barnes N, Boulet LP, Edwards D, Fowler A, et al. Efficacy and safety of once-daily single-inhaler triple therapy (FF/UMEC/VI) versus FF/VI in patients with inadequately controlled asthma (CAPTAIN): a double-blind, randomised, phase 3A trial. Lancet Respiratory Medicine 2021;9(1):69-84. [DOI: 10.1016/S2213-2600(20)30389-1] [PMID: ] - DOI - PubMed
Lin 2015 {published data only}
Lotvall 2014 {published and unpublished data}
-
- Lötvall J, Bleecker ER, Busse WW, O'Byrne PM, Woodcock A, Kerwin EM, et al. Efficacy and safety of fluticasone furoate 100 μg once-daily in patients with persistent asthma: a 24-week placebo and active-controlled randomised trial. Respiratory Medicine 2014;108(1):41-9. [DOI: 10.1016/j.rmed.2013.11.009] [PMID: ] - DOI - PubMed
Mansfield 2017 {published data only}
-
- Mansfield L, Yiu G, Sakov A, Liu S, Caracta C. A 6-month safety and efficacy study of fluticasone propionate and fluticasone propionate/salmeterol multidose dry powder inhalers in persistent asthma. Allergy & Asthma Proceedings 2017;38(4):264-76. [DOI: 10.2500/aap.2017.38.4061] [PMID: ] - DOI - PubMed
Murphy 2015 {published data only}
Nathan 2010 {published data only}
-
- Nathan RA, Nolte H, Pearlman DS. Twenty-six-week efficacy and safety study of mometasone furoate/formoterol 200/10 microg combination treatment in patients with persistent asthma previously receiving medium-dose inhaled corticosteroids. Allergy & Asthma Proceedings 2010;31(4):269-79. [DOI: 10.2500/aap.2010.31.3364] [PMID: ] - DOI - PubMed
O'Byrne 2014 {published data only}
Paggiaro 2016b {published data only}
-
- Paggiaro P, Halpin DM, Buhl R, Engel M, Zubek VB, Blahova Z, et al. The effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. Journal of Allergy and Clinical Immunology: In Practice 2016;4(1):104-13. [DOI: 10.1016/j.jaip.2015.08.017] [PMID: ] - DOI - PubMed
Papi 2007 {published data only}
Pedersen 2017 {published data only}
Pertseva 2013 {published data only}
-
- Pertseva T, Dissanayake S, Kaiser K. Superiority of fluticasone propionate/formoterol fumarate versus fluticasone propionate alone in patients with moderate-to-severe asthma: a randomised controlled trial. Current Medical Research and Opinion 2013;29(10):1357-69. [DOI: 10.1185/03007995.2013.825592] [PMID: ] - DOI - PubMed
Peters 2008 {published data only}
Peters 2016 {published data only}
Sher 2017 {published data only}
Spector 2012 {published data only}
Stempel 2016 {published data only}
Stirbulov 2012 {published data only}
-
- Stirbulov R, Fritscher CC, Pizzichini E, Pizzichini MM. Evaluation of the efficacy and safety of a fixed-dose, single-capsule budesonide-formoterol combination in uncontrolled asthma: a randomized, double-blind, multicenter, controlled clinical trial. Brazilian Journal of Pulmonology 2012;38(4):431-7. [DOI: 10.1590/s1806-37132012000400004] [PMID: ] - DOI - PubMed
van Zyl‐Smit 2020 {published data only}
-
- Zyl-Smit RN, Krüll M, Gessner C, Gon Y, Noga O, Richard A, et al. Once-daily mometasone plus indacaterol versus mometasone or twice-daily fluticasone plus salmeterol in patients with inadequately controlled asthma (PALLADIUM): a randomised, double-blind, triple-dummy, controlled phase 3 study. Lancet Respiratory Medicine 2020;8(10):987-99. [DOI: 10.1016/S2213-2600(20)30178-8] [PMID: ] - DOI - PubMed
Weinstein 2010 {published data only}
-
- Weinstein SF, Corren J, Murphy K, Nolte H, White M. Twelve-week efficacy and safety study of mometasone furoate/formoterol 200/10 microg and 400/10 microg combination treatments in patients with persistent asthma previously receiving high-dose inhaled corticosteroids. Allergy & Asthma Proceedings 2010;31(4):280-9. [DOI: 10.2500/aap.2010.31.3381] [PMID: ] - DOI - PubMed
Woodcock 2013 {published data only}
-
- Woodcock A, Bleecker ER, Lötvall J, O'Byrne PM, Bateman ED, Medley H, et al. Efficacy and safety of fluticasone furoate/vilanterol compared with fluticasone propionate/salmeterol combination in adult and adolescent patients with persistent asthma: a randomized trial. Chest 2013;144(4):1222-9. [DOI: 10.1378/chest.13-0178] [PMID: ] - DOI - PMC - PubMed
Woodcock 2014 {published data only}
-
- Woodcock A, Lötvall J, Busse WW, Bateman ED, Stone S, Ellsworth A, et al. Efficacy and safety of fluticasone furoate 100 μg and 200 μg once daily in the treatment of moderate-severe asthma in adults and adolescents: a 24-week randomised study. BMC Pulmonary Medicine 2014;14:113. [DOI: 10.1186/1471-2466-14-113] [PMID: ] - DOI - PMC - PubMed
Zangrilli 2011 {published data only}
-
- Zangrilli J, Mansfield LE, Uryniak T, O'Brien CD. Efficacy of budesonide/formoterol pressurized metered-dose inhaler versus budesonide pressurized metered-dose inhaler alone in Hispanic adults and adolescents with asthma: a randomized, controlled trial. Annals of Allergy, Asthma & Immunology 2011;107(3):258-65. [DOI: 10.1016/j.anai.2011.05.024] [PMID: ] - DOI - PubMed
References to studies excluded from this review
Amar 2016 {published data only}
Antilla 2014 {published data only}
-
- Antilla M, Castro F, Cruz Á, Rubin A, Rosário N, Stelmach R. Efficacy and safety of the single-capsule combination of fluticasone/formoterol in patients with persistent asthma: a non-inferiority trial. Brazilian Journal of Pulmonology 2014;40(6):599-608. [DOI: 10.1590/S1806-37132014000600003] [PMID: ] - DOI - PMC - PubMed
Barnes 2013 {published data only}
Bateman 2011 {published data only}
-
- Bateman ED, Kornmann O, Schmidt P, Pivovarova A, Engel M, Fabbri LM. Tiotropium is noninferior to salmeterol in maintaining improved lung function in B16-Arg/Arg patients with asthma. Journal of Allergy and Clinical Immunology 2011;128(2):315-22. [DOI: 10.1016/j.jaci.2011.06.004] [PMID: ] - DOI - PubMed
Berger 2010 {published data only}
-
- Berger WE, Bleecker ER, O'Dowd L, Miller CJ, Mezzanotte W. Efficacy and safety of budesonide/formoterol pressurized metered-dose inhaler: randomized controlled trial comparing once- and twice-daily dosing in patients with asthma. Allergy & Asthma Proceedings 2010;31(1):49-59. [DOI: 10.2500/aap.2010.31.3309] [PMID: ] - DOI - PubMed
Bernstein 2018 {published data only}
Bodzenta‐Lukaszyk 2011 {published data only}FLT3501
-
- Bodzenta-Lukaszyk A, Dymek A, McAulay K, Mansikka H. Fluticasone/formoterol combination therapy is as effective as fluticasone/salmeterol in the treatment of asthma, but has a more rapid onset of action: an open-label, randomized study. BMC Pulmonary Medicine 2011;11:28. [DOI: 10.1186/1471-2466-11-28] [PMID: ] - DOI - PMC - PubMed
Bodzenta‐Lukaszyk 2013 {published data only}FLT3505
-
- Bodzenta-Lukaszyk A, Noord J, Schröder-Babo W, McAulay K, McIver T. Efficacy and safety profile of fluticasone/formoterol combination therapy compared to its individual components administered concurrently in asthma: a randomised controlled trial. Current Medical Research and Opinion 2013;29(5):579-88. [DOI: 10.1185/03007995.2013.772506] [PMID: ] - DOI - PubMed
Boyd 1995 {published data only}
-
- Boyd G. Salmeterol xinafoate in asthmatic patients under consideration for maintenance oral corticosteroid therapy. European Respiratory Journal 1995;8(9):1494-8. [PMID: ] - PubMed
Busse 2013 {published data only}
-
- Busse WW, O'Byrne PM, Bleecker ER, Lötvall J, Woodcock A, Andersen L, et al. Safety and tolerability of the novel inhaled corticosteroid fluticasone furoate in combination with the β2 agonist vilanterol administered once daily for 52 weeks in patients >=12 years old with asthma: a randomised trial. Thorax 2013;68(6):513-20. [DOI: ] [PMID: ] - PMC - PubMed
Busse 2018 {published data only}
Corradi 2016 {published data only}
Devillier 2018 {published data only}
-
- Devillier P, Humbert M, Boye A, Zachgo W, Jacques L, Nunn C, et al. Efficacy and safety of once-daily fluticasone furoate/vilanterol (FF/VI) versus twice-daily inhaled corticosteroids/long-acting β2-agonists (ICS/LABA) in patients with uncontrolled asthma: an open-label, randomized, controlled trial. Respiratory Medicine 2018;141:111-20. [DOI: 10.1016/j.rmed.2018.06.009] [PMID: ] - DOI - PubMed
Hamelmann 2017 {published data only}
Hoshino 2016 {published data only}
Kerwin 2009 {published data only}
-
- Kerwin EM, Oppenheimer JJ, LaForce C, Parasuraman B, Miller CJ, O'Dowd L, et al. Efficacy and tolerability of once-daily budesonide/formoterol pressurized metered-dose inhaler in adults and adolescents with asthma previously stable with twice-daily budesonide/formoterol dosing. Annals of Allergy, Asthma & Immunology 2009;103(1):62-72. [DOI: 10.1016/S1081-1206(10)60145-7] [PMID: ] - DOI - PubMed
Kerwin 2017 {published data only}
-
- Kerwin EM, Gillespie M, Song S, Steinfeld J. Randomized, dose-ranging study of a fluticasone propionate multidose dry powder inhaler in adolescents and adults with uncontrolled asthma not previously treated with inhaled corticosteroids. Journal of Asthma 2017;54(1):89-98. [DOI: 10.1080/02770903.2016.1193870] [PMID: ] - DOI - PubMed
Koenig 2008 {published data only}
Kornmann 2020 {published data only}
-
- Kornmann O, Mucsi J, Kolosa N, Bandelli L, Sen B, Satlin LC, et al. Efficacy and safety of inhaled once-daily low-dose indacaterol acetate/mometasone furoate in patients with inadequately controlled asthma: phase III randomised QUARTZ study findings. Respiratory Medicine 2020;161:105809. [DOI: 10.1016/j.rmed.2019.105809] [PMID: ] - DOI - PubMed
Lenney 2013 {published data only}
-
- Lenney W, McKay AJ, Tudur Smith C, Williamson PR, James M, Price D. Management of Asthma in School age Children On Therapy (MASCOT): a randomised, double-blind, placebo-controlled, parallel study of efficacy and safety. Health Technology Assessment 2013;17(4):1-218. [DOI: 10.3310/hta17040] [PMID: ] - DOI - PMC - PubMed
Lötvall 2014 {published data only}
-
- Lötvall J, Bateman ED, Busse WW, O'Byrne PM, Woodcock A, Toler WT, et al. Comparison of vilanterol, a novel long-acting beta2 agonist, with placebo and a salmeterol reference arm in asthma uncontrolled by inhaled corticosteroids. Journal of Negative Results in Biomedicine 2014;13(1):9. [DOI: 10.1186/1477-5751-13-9] [PMID: ] - DOI - PMC - PubMed
Maspero 2010 {published data only}
Murphy 2012 {published data only}
-
- Murphy KR, Uryniak T, Martin UJ, Zangrilli J. The effect of budesonide/formoterol pressurized metered-dose inhaler on predefined criteria for worsening asthma in four different patient populations with asthma. Drugs in R&D 2012;12(1):9-14. [DOI: 10.2165/11630600-000000000-00000] [SPRUCE] [PMID: ] - DOI - PMC - PubMed
Murphy 2015x {published data only}
-
- Murphy KR, Uryniak T, Martin UJ, Zangrilli J. The effect of budesonide/formoterol pressurized metered-dose inhaler on predefined criteria for worsening asthma in four different patient populations with asthma. Drugs in R&D 2012;12(1):15. [DOI: 10.2165/11630600-000000000-00000] [PMID: ] - DOI - PMC - PubMed
Nathan 2012 {published data only}
NCT00529529 {unpublished data only}
-
- Novartis. Safety of indacaterol in patients (≥ 12 years) with moderate to severe persistent asthma. https://clinicaltrials.gov/ct2/show/NCT00529529 (accessed 29 August 2011). [STUDY ID: CQAB149B2338]
NCT01001364 {unpublished data only}
-
- Eurofarma Laboratorios. A Comparative Study Between Foraseq And Formoterol/Budesonide Inhalation Capsules in Patients With Asthma (CAINAS). https://clinicaltrials.gov/ct2/show/NCT01001364 (accessed 28 June 2011). [STUDY ID: EF-091]
NCT01202084 {unpublished data only}
-
- Eurofarma Laboratorios. A study comparative of formoterol/fluticasone Foraseq® and fluticasone in asthma patients. https://www.clinicaltrials.gov/ct2/show/NCT01202084 (accessed 18 June 2015). [STUDY ID: EF 065]
NCT01609478 {unpublished data only}
-
- Novartis. Efficacy, safety and pharmacokinetics of indacaterol acetate in patients with persistent asthma. https://clinicaltrials.gov/ct2/show/NCT01609478 (accessed 27 January 2015). [STUDY ID: CQMF149E2203]
NCT01720069 {unpublished data only}
-
- Vectura Limited. Clinical study to evaluate the efficacy and safety of VR506 using a new inhaler for the treatment of asthma. https://clinicaltrials.gov/ct2/show/NCT01720069 (accessed 21 April 2020). [STUDY ID: VR506/2/004]
NCT01845025 {unpublished data only}
-
- Novartis. Study of safety of foradil in patients with persistent asthma. https://clinicaltrials.gov/ct2/show/NCT01845025 (accessed March 2017). [STUDY ID: CFOR258D2416]
NCT02094937 {unpublished data only}
-
- GlaxoSmithKline. A study to compare the efficacy and safety of fluticasone furoate (FF) 100 mcg once daily with fluticasone propionate (FP) 250 mcg twice daily (BD) and FP 100 mcg BD in well-controlled asthmatic Japanese subjects. https://www.clinicaltrials.gov/ct2/show/NCT02094937 (accessed 25 May 2017). [GSK STUDY ID: 201135]
NCT04677959 {unpublished data only}
-
- Teva Branded Pharmaceutical. A 24-week treatment study to compare standard of care versus the eMDPI DS in patients 13 years or older with asthma (CONNECT2). https://clinicaltrials.gov/ct2/show/NCT04677959 (accessed 18 July 2022). [STUDY ID: FSS-AS-40139]
Ohta 2015 {published data only}
-
- Ohta K, Ichinose M, Tohda Y, Engel M, Moroni-Zentgraf P, Kunimitsu S, et al. Long-term once-daily tiotropium Respimat® is well tolerated and maintains efficacy over 52 weeks in patients with symptomatic asthma in Japan: a randomised, placebo-controlled study. PLoS One 2015;10(4):e0124109. [DOI: 10.1371/journal.pone.0124109] [PMID: ] - DOI - PMC - PubMed
Paggiaro 2016a {published data only}
-
- Paggiaro P, Halpin DM, Buhl R, Engel M, Zubek VB, Blahova Z, et al. The effect of tiotropium in symptomatic asthma despite low- to medium-dose inhaled corticosteroids: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2016;4(1):104-13. [DOI: 10.1016/j.jaip.2015.08.017] [PMID: ] - DOI - PubMed
Peters 2010 {published data only}
Renzi 2010 {published data only}
Tashkin 2016 {published data only}
-
- Tashkin DP, Moore GE, Trudo F, DePietro M, Chipps BE. Assessment of consistency of fixed airflow obstruction status during budesonide/formoterol treatment and its effects on treatment outcomes in patients with asthma. Journal of Allergy and Clinical Immunology 2016;4(4):705-12. [DOI: 10.1016/j.jaip.2016.02.014] [PMID: ] - DOI - PubMed
Wechsler 2016 {published data only}
-
- Wechsler ME, Yawn BP, Fuhlbrigge AL, Pace WD, Pencina MJ, Doros G, et al. Anticholinergic vs long-acting β-agonist in combination with Inhaled corticosteroids in Black adults with asthma: the BELT Randomized Clinical Trial. JAMA 2015;314(16):1720-30. [DOI: 10.1001/jama.2015.13277] [PMID: ] - DOI - PubMed
Wechsler 2019 {published data only}
Weinstein 2019 {published data only}
Woodcock 2017 {published data only}
-
- Woodcock A, Vestbo J, Bakerly ND, New J, Gibson JM, McCorkindale S, et al. Effectiveness of fluticasone furoate plus vilanterol on asthma control in clinical practice: an open-label, parallel group, randomised controlled trial. Lancet 2017;390(10):32397-8. [DOI: 10.1016/S0140-6736(17)32397-8] [PMID: ] - DOI - PubMed
References to ongoing studies
NCT03248128 {unpublished data only}
-
- GlaxoSmithKline. Safety and efficacy study of fluticasone furoate/vilanterol (FF/VI) fixed dose combination (FDC) compared to FF alone in subjects with asthma. https://clinicaltrials.gov/ct2/show/NCT03248128 (first received 14 August 2017). [GSK STUDY ID: 107116]
NCT03387241 {unpublished data only}
-
- Mundipharma. Efficacy of FLUTIFORM® vs Seretide® in moderate to severe persistent asthma in subjects aged ≥12 years. https://clinicaltrials.gov/ct2/show/NCT03387241 (first received 2 January 2018). [STUDY ID: FLT13-CN-301]
NCT04191434 {unpublished data only}
-
- EMS. Efficacy and safety of flamboyant 125/12 association in the treatment of adults with moderate asthma. https://clinicaltrials.gov/ct2/show/NCT04191434 (first received 9 December 2019). [STUDY ID: EMS0219 - FLAMBOYANT125/12]
NCT04191447 {unpublished data only}
-
- EMS. Efficacy and safety of flamboyant 200/12 association in the treatment of adults with severe asthma. https://clinicaltrials.gov/ct2/show/NCT04191447 (first received 9 December 2019). [STUDY ID: EMS0319 - FLAMBOYANT200/12]
NCT05202262 (VATHOS) {unpublished data only}
-
- AstraZeneca. A 24-week efficacy and safety study to assess budesonide and formoterol fumarate metered dose inhaler in adult and adolescent participants with inadequately controlled asthma (VATHOS). https://clinicaltrials.gov/ct2/show/NCT05202262 (first received 21 January 2022). [STUDY ID: D5982C00006]
Additional references
Anderson 2015
-
- Anderson DE, Kew KM, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus the same dose of ICS alone for adults with asthma. Cochrane Database of Systematic Reviews 2015, Issue 8. Art. No: CD011397. [DOI: 10.1002/14651858.CD011397.pub2] [PMID: ] - DOI - PMC - PubMed
Barnes 1993
Barnes 2010
Bateman 2014
Beasley 2019
Bernstein 2018
Brignardello‐Petersen 2018
BTS/SIGN 2019
-
- British Thoracic Society. BTS/SIGN British Guideline on the Management of Asthma 2019. www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/ (accessed prior to 27 March 2020).
Buhl 2018
Chipps 2020
-
- Chipps B, Taylor B, Bayer V, Shaikh A, Mosnaim G, Trevor J, et al. Relative efficacy and safety of inhaled corticosteroids in patients with asthma: systematic review and network meta-analysis. Annals of Allergy, Asthma & Immunology 2020;125(2):163-70. [DOI: 10.1016/j.anai.2020.04.006] [PMID: ] - DOI - PubMed
Cochrane Airways 2019
-
- Cochrane Airways Trials Register. www.airways.cochrane.org/trials-register (accessed 7 May 2019).
Dechartres 2013
Deeks 2022
-
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane, 2022. Available from www.training.cochrane.org/handbook.
Derom 1992
Dias 2010
-
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29(7-8):932-44. [DOI: ] [PMID: ] - PubMed
Dias 2013
Dias 2018
-
- Dias S, Ades AE, Welton NJ, Jansen JP, Sutton AJ. Network Meta-Analysis for Decision-Making. Hoboken, New Jersey: John Wiley & Sons Ltd, 2018. [ISBN 9781118647509]
Ducharme 2010a
-
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled corticosteroids versus same dose inhaled corticosteroids for chronic asthma in adults and children. Cochrane Database of Systematic Reviews 2010, Issue 5. Art. No: CD005535. [DOI: 10.1002/14651858.CD005535.pub2] - DOI - PMC - PubMed
Ducharme 2010b
-
- Ducharme FM, Ni Chroinin M, Greenstone I, Lasserson TJ. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database of Systematic Reviews 2010, Issue 4. Art. No: CD005533. [DOI: 10.1002/14651858.CD005533.pub2] [PMID: ] - DOI - PMC - PubMed
Egger 1997
EPR‐4 2020
-
- The National Heart, Lung, and Blood Institute. Update on selected topics in asthma management: a report from the National Asthma Education and Prevention Program Coordinating Committee Expert Panel Working Group. www.nhlbi.nih.gov/about/advisory-and-peer-review-committees/national-ast... (accessed 14 July 2020).
FDA 2016
-
- US Food and Drug Administration. What is a serious adverse event? www.fda.gov/safety/reporting-serious-problems-fda/what-serious-adverse-e... ;(accessed 25 August 2020).
GeMTC package [Computer program]
-
- gemtc. Valkenhoef G, Kuiper J, Version Version 1.0-1. Groningen, Netherlands: Gert van Valkenhoef, May 14, 2021. https://CRAN.R-project.org/package=gemtc.
GINA 2022
-
- Global Initiative for Asthma. 2022 GINA Report, Global Strategy for Asthma Management and Prevention. https://ginasthma.org/wp-content/uploads/2022/07/GINA-Main-Report-2022-F... (accessed 21 September 2022).
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed 17 January 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Guyatt 2011
Guyatt 2017
-
- Guyatt GH, Ebrahim S, Alonso-Coello P, Johnston BC, Mathioudakis AG, Briel M, et al. GRADE guidelines 17: assessing the risk of bias associated with missing participant outcome data in a body of evidence. Journal of Clinical Epidemiology 2017;87:14-22. [PMID: ] - PubMed
Haahtela 1991
-
- Haahtela T, Järvinen M, Kava T, Kiviranta K, Koskinen S, Lehtonen K, et al. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. New England Journal of Medicine 1991;325(6):388-92. [DOI: 10.1056/NEJM199108083250603] [PMID: ] - DOI - PubMed
Higgins 2019
-
- Higgins JP, Savović J, Page MJ, Elbers RG, Sterne JA. Chapter 8: Assessing risk of bias in a randomized trial. In Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s), Cochrane Handbook for Systematic Reviews of Interventions version 6.1 (updated September 2019). The Cochrane Collaboration. Available from www.training.cochrane.org/handbook.
Holt 2001
-
- Holt S, Herxheimer A, Suder A, Weatherall M, Cheng S, Shirtcliffe P, et al. Dose-response relation of inhaled fluticasone propionate in adolescents and adults with asthma: meta-analysis commentary: dosage needs systematic and critical review. BMJ 2001;323(7307):253-6. [DOI: 10.1136/bmj.323.7307.253] [PMID: ] - DOI - PMC - PubMed
Juniper 1994
Juniper 2005
-
- Juniper EF, Svensson K, Mörk AC, Ståhl E. Measurement properties and interpretation three shortened versions of the asthma control questionnaire. Respiratory Medicine 2005;99(5):553-8. [PMID: ] - PubMed
Kerstjens 2012
Kerstjens 2015
-
- Kerstjens HA, Casale TB, Bleecker ER, Meltzer EO, Pizzichini E, Schmidt O, et al. Tiotropium or salmeterol as add-on therapy to inhaled corticosteroids for patients with moderate symptomatic asthma: two replicate, double-blind, placebo-controlled, parallel-group, active-comparator, randomised trials. Lancet Respiratory Medicine 2015;3(5):367-76. [DOI: 10.1016/S2213-2600(15)00031-4] [PMID: ] - DOI - PubMed
Kew 2015
-
- Kew KM, Evans DJ, Allison DE, Boyter AC. Long-acting muscarinic antagonists (LAMA) added to inhaled corticosteroids (ICS) versus addition of long-acting beta2-agonists (LABA) for adults with asthma. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No: CD011438. [DOI: 10.1002/14651858.CD011438.pub2] [PMID: ] - DOI - PMC - PubMed
Kips 2001
Lipworth 2014
Marshall 2018
Masoli 2004
McDonald 2017
-
- McDonald S, Noel-Storr AH, Thomas J. Harnessing the efficiencies of machine learning and Cochrane Crowd to identify randomised trials for individual Cochrane reviews. In: Global Evidence Summit; 2017 September 13-16; Cape Town, South Africa. 2017.
McGowan 2016
Moher 2009
Neupane 2014
NICE 2018
-
- National Institute for Health and Care Excellence. Inhaled corticosteroid doses for NICE’s asthma guideline. www.nice.org.uk/guidance/ng80/resources/inhaled-corticosteroid-doses-pdf... (accessed prior to 27 March 2020).
Noel‐Storr 2018
-
- Noel-Storr AH, Project Transform team. Cochrane Crowd: new ways of working together to produce health evidence. In: Evidence Live; 2018 June 18-20; Oxford, UK. 2018.
Nurmagambetov 2018
Nüesch 2010
OpenBUGS [Computer program]
-
- OpenBUGS. Thomas A, Version 3.2.3. Helsinki: Wiki, accessed prior to 27 March 2020. Available at www.openbugs.net/w/FrontPage.
Paik 2018
Peters 2010
Petsky 2018
Phillippo 2018
Phillippo 2019
-
- Phillippo DM, Dias S, Welton NJ, Caldwell DM, Taske N, Ades AE. Threshold analysis as an alternative to GRADE for assessing confidence in guideline recommendations based on network meta-analyses. Annals of Internal Medicine 2019;170(8):538-46. [DOI: 10.7326/M18-3542] [PMC6739230] [PMID: ] - DOI - PMC - PubMed
R [Computer program]
-
- R: A Language and Environment for Statistical Computing. R Core Team, Version 4.1.1. Vienna, Austria: R Foundation for Statistical Computing, 10 August 2021. https://www.R-project.org.
Review Manager 2020 [Computer program]
-
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
RoB 2 Excel tool [Computer program]
-
- Risk of Bias 2 tool. London, England: Cochrane, 22 August 2019. https://sites.google.com/site/riskofbiastool/welcome/rob-2-0-tool/curren....
Rodrigo 2015
Röver 2021
Schatz 2006
-
- Schatz M, Sorkness CA, Li JT, Marcus P, Murray JJ, Nathan RA, et al. Asthma Control Test: reliability, validity, and responsiveness in patients not previously followed by asthma specialists. Journal of Allergy and Clinical Immunology 2006;117(3):549-56. [DOI: 10.1016/j.jaci.2006.01.011] [PMID: ] - DOI - PubMed
Schünemann 2019
-
- Schünemann HJ, Higgins JP, Vist GE, Glasziou P, Akl EA, Skoetz N, et al. Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration. Available from www.training.cochrane.org/handbook.
Shimoda 2016
Sobieraj 2018
-
- Sobieraj DM, Weeda ER, Nguyen E, Coleman CI, White CM, Lazarus SC, et al. Association of inhaled corticosteroids and long-acting β-agonists as controller and quick relief therapy with exacerbations and symptom control in persistent asthma: a systematic review and meta-analysis. JAMA 2018;319(14):1485-96. [DOI: 10.1001/jama.2018.2769] [PMID: ] - DOI - PMC - PubMed
Spiegelhalter 2002
-
- Spiegelhalter DJ, Best NG, Carlin BP, Van der Linde A. Bayesian measures of model complexity and fit. Series B (Statistical Methodology). Journal of the Royal Statistical Society 2002;64(4):583-639. [DOI: 10.1111/1467-9868.00353] - DOI
Sterne 2019
Thomas 2011
Thomas 2017
-
- Thomas J, Noel-Storr AH, Marshall I, Wallace B, McDonald S, Mavergames C, et al. Living systematic review network. Living systematic reviews: 2. Combining human and machine effort. Journal of Clinical Epidemiology 2017;91:31-7. - PubMed
Turner 2015
van Valkenhoef 2016
Wechsler 2015
-
- Wechsler ME, Yawn BP, Fuhlbrigge AL, Pace WD, Pencina MJ, Doros G, et al. Anticholinergic vs long-acting beta-agonist in combination with inhaled corticosteroids in black adults with asthma: the BELT randomized clinical trial. JAMA 2015;314(16):1720-30. [DOI: 10.1001/jama.2015.13277] [PMID: ] - DOI - PubMed
Yepes‐Nuñez 2019
Zahran 2018
Zhang 2014
-
- Zhang L, Prietsch SO, Ducharme FM. Inhaled corticosteroids in children with persistent asthma: effects on growth. Evidence-Based Child Health 2014;9(4):829-930. - PubMed
References to other published versions of this review
Oba 2020
-
- Oba Y, Patel T, Anwer S, Maduke T, Dias S. Addition of long-acting beta2 agonists or long-acting muscarinic antagonists versus doubling the dose of inhaled corticosteroids (ICS) in adolescents and adults with uncontrolled asthma with medium dose ICS: a systematic review and network meta-analysis. Cochrane Database of Systematic Reviews 2020, Issue 11. Art. No: CD013797. [DOI: 10.1002/14651858.CD013797] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

