Tirzepatide in Hispanic/Latino Patients With Type 2 Diabetes: A Subgroup Analysis of the SURPASS Program
- PMID: 37602701
- PMCID: PMC10795909
- DOI: 10.1210/clinem/dgad495
Tirzepatide in Hispanic/Latino Patients With Type 2 Diabetes: A Subgroup Analysis of the SURPASS Program
Abstract
Context: Efficacy and safety of tirzepatide, a once-weekly glucose-dependent insulinotropic polypeptide and glucagon-like peptide-1 receptor agonist, have been studied in patients with type 2 diabetes in the global phase 3 SURPASS program.
Objective: This work aimed to assess the efficacy and safety of tirzepatide in Hispanic/Latino and non-Hispanic/Latino patients in SURPASS-1 to -4 clinical trials.
Methods: A total of 5679 patients were included, 2895 of self-reported Hispanic/Latino ethnicity, in this exploratory analysis of SURPASS-1 to -4 trial data. Interventions included tirzepatide 5, 10, or 15 mg, placebo, or active comparator (semaglutide 1 mg, insulin degludec, and insulin glargine). Change in glycated hemoglobin A1c (HbA1c) and body weight from baseline to week 40 (SURPASS-1 and -2) and to week 52 (SURPASS-3 and -4), and other efficacy and safety outcomes were evaluated within Hispanic/Latino and non-Hispanic/Latino subgroups.
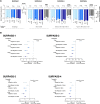
Results: Among Hispanic/Latino and non-Hispanic/Latino patients treated with tirzepatide, respectively, HbA1c decreased significantly from baseline, ranging from 1.9% to 2.7% and 1.7% to 2.5%, and body weight decreased significantly from baseline, ranging from 5.3 kg to 12.4 and 6.5 kg to 17.1 kg (both P < .05) vs comparators across all trials. Subgroup trends were consistent with the overall trial populations. Treatment-emergent adverse events were reported in similar proportions across the subgroups and were primarily gastrointestinal disorders. The incidence of hypoglycemia was low.
Conclusion: Tirzepatide significatively reduced HbA1c and body weight in Hispanic/Latino and non-Hispanic/Latino patients. Tirzepatide was generally well tolerated in both subgroups. Efficacy and safety trends were comparable between subgroups and within the overall trial populations.
Keywords: GIP and GLP-1 receptor agonist; Hispanic/Latino; glycemic control; incretin therapy; tirzepatide; type 2 diabetes.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures

References
-
- Gallardo-Rincon H, Cantoral A, Arrieta A, et al. Review: type 2 diabetes in Latin America and the Caribbean: regional and country comparison on prevalence, trends, costs and expanded prevention. Prim Care Diabetes. 2021;15(2):352‐359. - PubMed
-
- Mainous AG III, Diaz VA, Koopman RJ, Everett CJ. Quality of care for Hispanic adults with diabetes. Fam Med. 2007;39(5):351‐356. - PubMed
-
- Kirk JK, Passmore LV, Bell RA, et al. Disparities in A1C levels between Hispanic and non-Hispanic white adults with diabetes: a meta-analysis. Diabetes Care. 2008;31(2):240‐246. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

