Stage 4N neuroblastoma before and during the era of anti-GD2 immunotherapy
- PMID: 37602920
- PMCID: PMC11925214
- DOI: 10.1002/ijc.34693
Stage 4N neuroblastoma before and during the era of anti-GD2 immunotherapy
Abstract
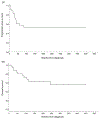
Patients with stage 4N neuroblastoma (distant metastases limited to lymph nodes) stand out as virtually the only survivors of high-risk neuroblastoma (HR-NB) before myeloablative therapy (MAT) and immunotherapy with anti-GD2 monoclonal antibodies (mAbs) became standard. Because no report presents more recent results with 4N, we analyzed our large 4N experience. All 51 pediatric 4N patients (<18 years old) diagnosed 1985 to 2021 were reviewed. HR-NB included MYCN-nonamplified 4N diagnosed at age ≥18 months and MYCN-amplified 4N. Among 34 MYCN-nonamplified high-risk patients, 20 are relapse-free 1.5+ to 37.5+ (median 12.5+) years post-diagnosis, including 13 without prior MAT and 5 treated with little (1 cycle; n = 2) or no mAb (n = 3), while 14 patients (7 post-MAT, 8 post-mAbs) relapsed (all soft tissue). Of 15 MYCN-amplified 4N patients, 7 are relapse-free 2.1+ to 26.4+ (median 11.6+) years from the start of chemotherapy (all received mAbs; 3 underwent MAT) and 4 are in second remission 4.2+ to 21.8+ years postrelapse (all soft tissue). Statistical analyses showed no significant association of survival with either MAT or mAbs for MYCN-nonamplified HR-NB; small numbers prevented these analyses for MYCN-amplified patients. The two patients with intermediate-risk 4N (14-months-old) are relapse-free 7+ years postresection of primary tumors; distant disease spontaneously regressed. The natural history of 4N is marked by NB confined to soft tissue without early relapse in bones or bone marrow, where mAbs have proven efficacy. These findings plus curability without MAT, as seen elsewhere and at our center, support consideration of treatment reduction for MYCN-nonamplified 4N.
Keywords: immunotherapy; metastases; neuroblastoma; prognosis; radiotherapy.
© 2023 UICC.
Conflict of interest statement
Figures
References
-
- Irwin MS, Naranjo A, Zhang FF, Cohn SL, London WB, Gastier-Foster JM, Ramirez NC, Pfau R, Reshmi S, Wagner E, Nuchtern J, Asgharzadeh S, Shimada H, Maris JM, Bagatell R, Park JR, Hogarty MD. Revised Neuroblastoma Risk Classification System: A Report From the Children’s Oncology Group. J Clin Oncol 2021;39: 3229–41. - PMC - PubMed
-
- Twist CJ, Schmidt ML, Naranjo A, London WB, Tenney SC, Marachelian A, Shimada H, Collins MH, Esiashvili N, Adkins ES, Mattei P, Handler M, Katzenstein H, Attiyeh E, Hogarty MD, Gastier-Foster J, Wagner E, Matthay KK, Park JR, Maris JM, Cohn SL. Maintaining Outstanding Outcomes Using Response- and Biology-Based Therapy for Intermediate-Risk Neuroblastoma: A Report From the Children’s Oncology Group Study ANBL0531. J Clin Oncol 2019;37: 3243–55. - PMC - PubMed
-
- Yu AL, Gilman AL, Ozkaynak MF, London WB, Kreissman SG, Chen HX, Smith M, Anderson B, Villablanca JG, Matthay KK, Shimada H, Grupp SA, Seeger R, Reynolds CP, Buxton A, Reisfeld RA, Gillies SD, Cohn SL, Maris JM, Sondel PM, Children’s Oncology G. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med 2010;363: 1324–34. - PMC - PubMed
-
- Cheung NK, Cheung IY, Kushner BH, Ostrovnaya I, Chamberlain E, Kramer K, Modak S. Murine anti-GD2 monoclonal antibody 3F8 combined with granulocyte-macrophage colony-stimulating factor and 13-cis-retinoic acid in high-risk patients with stage 4 neuroblastoma in first remission. J Clin Oncol 2012;30: 3264–70. - PMC - PubMed
-
- Ladenstein R, Potschger U, Pearson ADJ, Brock P, Luksch R, Castel V, Yaniv I, Papadakis V, Laureys G, Malis J, Balwierz W, Ruud E, Kogner P, Schroeder H, de Lacerda AF, Beck-Popovic M, Bician P, Garami M, Trahair T, Canete A, Ambros PF, Holmes K, Gaze M, Schreier G, Garaventa A, Vassal G, Michon J, Valteau-Couanet D, Group SEN. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol 2017;18: 500–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical