cGAS-STING Pathway Activation during Trypanosoma cruzi Infection Leads to Tissue-Dependent Parasite Control
- PMID: 37603014
- PMCID: PMC10783805
- DOI: 10.4049/jimmunol.2300373
cGAS-STING Pathway Activation during Trypanosoma cruzi Infection Leads to Tissue-Dependent Parasite Control
Abstract
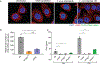
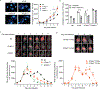
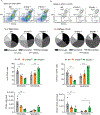
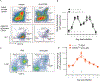
Host cell invasion by Trypanosoma cruzi is a markedly silent process, with limited host transcriptional changes indicative of innate immune recognition, except for a modest type I IFN (IFN-I) response. In this study, we show that T. cruzi-induced IFN-β production was nearly abolished in primary murine cGAS-/- or stimulator of IFN genes (STING)-deficient (STINGGt) macrophages and fibroblasts. T. cruzi infection did not impact the ability of IFN-regulatory factor reporter macrophages to respond to classical cGAS-STING agonists, indicating that the limited IFN-β induction is not due to active parasite suppression. cGAS-/-, STINGGt, and IFN-α/β receptor-/- (IFNAR-/-) macrophages infected with T. cruzi yielded significantly higher numbers of amastigotes compared with wild-type macrophages; however, the impact of the STING pathway during infection in vivo is more complex. Despite an initial increase in parasite growth, STINGGt and IFNAR-/- mice ultimately had lower parasite burden in footpads as compared with wild-type mice, demonstrating a role for IFN-I expression in potentiating parasite growth at the infection site. STING pathway activation had little impact on parasite levels in the skeletal muscle; however, in the heart, cGAS-/- and STINGGt mice, but not IFNAR-/- mice, accumulated higher acute parasite loads, suggesting a protective role of STING sensing of T. cruzi in this organ that was independent of IFN-I. Together, these results demonstrate that host cGAS-STING senses T. cruzi infection, enhancing parasite growth at the site of entry, and contributes to acute-phase parasite restriction in the heart, a major site of tissue damage in chronic T. cruzi infection.
Copyright © 2023 by The American Association of Immunologists, Inc.
Figures

Similar articles
-
STING Signaling Drives Production of Innate Cytokines, Generation of CD8+ T Cells and Enhanced Protection Against Trypanosoma cruzi Infection.Front Immunol. 2022 Jan 14;12:775346. doi: 10.3389/fimmu.2021.775346. eCollection 2021. Front Immunol. 2022. PMID: 35095849 Free PMC article.
-
Salmonella Induces the cGAS-STING-Dependent Type I Interferon Response in Murine Macrophages by Triggering mtDNA Release.mBio. 2022 Jun 28;13(3):e0363221. doi: 10.1128/mbio.03632-21. Epub 2022 May 23. mBio. 2022. PMID: 35604097 Free PMC article.
-
Delayed Activation of T Cells at the Site of Infection Facilitates the Establishment of Trypanosoma cruzi in Both Naive and Immune Hosts.mSphere. 2023 Feb 21;8(1):e0060122. doi: 10.1128/msphere.00601-22. Epub 2023 Jan 25. mSphere. 2023. PMID: 36695605 Free PMC article.
-
RNA viruses and the cGAS-STING pathway: reframing our understanding of innate immune sensing.Curr Opin Virol. 2022 Apr;53:101206. doi: 10.1016/j.coviro.2022.101206. Epub 2022 Feb 15. Curr Opin Virol. 2022. PMID: 35180533 Review.
-
The cGAS-STING Pathway in Bacterial Infection and Bacterial Immunity.Front Immunol. 2022 Jan 13;12:814709. doi: 10.3389/fimmu.2021.814709. eCollection 2021. Front Immunol. 2022. PMID: 35095914 Free PMC article. Review.
Cited by
-
The importance of persistence and dormancy in Trypanosoma cruzi infection and Chagas disease.Curr Opin Microbiol. 2025 Aug;86:102615. doi: 10.1016/j.mib.2025.102615. Epub 2025 Jun 6. Curr Opin Microbiol. 2025. PMID: 40482566 Review.
-
The transcriptome landscape of 3D-cultured placental trophoblasts reveals activation of TLR2 and TLR3/7 in response to low Trypanosoma cruzi parasite exposure.Front Microbiol. 2023 Sep 20;14:1256385. doi: 10.3389/fmicb.2023.1256385. eCollection 2023. Front Microbiol. 2023. PMID: 37799608 Free PMC article.
References
-
- Subbian S, Bandyopadhyay N, Tsenova L, O’Brien P, Khetani V, Kushner NL, Peixoto B, Soteropoulos P, Bader JS, Karakousis PC, Fallows D, and Kaplan G. 2013. Early innate immunity determines outcome of Mycobacterium tuberculosis pulmonary infection in rabbits. Cell Commun Signal 11: 60. - PMC - PubMed
-
- Pontiroli F, Dussurget O, Zanoni I, Urbano M, Beretta O, Granucci F, Ricciardi-Castagnoli P, Cossart P, and Foti M. 2012. The timing of IFNbeta production affects early innate responses to Listeria monocytogenes and determines the overall outcome of lethal infection. PLoS One 7: e43455. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

