Influence of DNP Polarizing Agents on Biochemical Processes: TEMPOL in Transient Ischemic Stroke
- PMID: 37603041
- PMCID: PMC10485885
- DOI: 10.1021/acschemneuro.3c00137
Influence of DNP Polarizing Agents on Biochemical Processes: TEMPOL in Transient Ischemic Stroke
Abstract
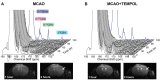
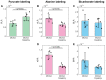
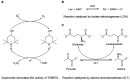
Hyperpolarization of 13C by dissolution dynamic nuclear polarization (dDNP) boosts the sensitivity of magnetic resonance spectroscopy (MRS), making possible the monitoring in vivo and in real time of the biochemical reactions of exogenously infused 13C-labeled metabolic tracers. The preparation of a hyperpolarized substrate requires the use of free radicals as polarizing agents. Although added at very low doses, these radicals are not biologically inert. Here, we demonstrate that the presence of the nitroxyl radical TEMPOL influences significantly the cerebral metabolic readouts of a hyperpolarized [1-13C] lactate bolus injection in a mouse model of ischemic stroke with reperfusion. Thus, the choice of the polarizing agent in the design of dDNP hyperpolarized MRS experiments is of great importance and should be taken into account to prevent or to consider significant effects that could act as confounding factors.
Keywords: Hyperpolarization; MRS; TEMPOL; dDNP; lactate; stroke.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

References
-
- Ardenkjaer-Larsen J.-H.; Boebinger G. S.; Comment A.; Duckett S.; Edison A. S.; Engelke F.; Griesinger C.; Griffin R. G.; Hilty C.; Maeda H.; Parigi G.; Prisner T.; Ravera E.; van Bentum J.; Vega S.; Webb A.; Luchinat C.; Schwalbe H.; Frydman L. Facing and Overcoming Sensitivity Challenges in Biomolecular NMR Spectroscopy. Angew. Chem., Int. Ed. 2015, 54 (32), 9162–9185. 10.1002/anie.201410653. - DOI - PMC - PubMed
-
- El Daraï T.; Jannin S. Sample Formulations for Dissolution Dynamic Nuclear Polarization. Chem. Phys. Rev. 2021, 2 (4), 041308.10.1063/5.0047899. - DOI
-
- Eichhorn T. R.; Takado Y.; Salameh N.; Capozzi A.; Cheng T.; Hyacinthe J.-N.; Mishkovsky M.; Roussel C.; Comment A. Hyperpolarization without Persistent Radicals for in Vivo Real-Time Metabolic Imaging. Proc. Natl. Acad. Sci. U.S.A. 2013, 110 (45), 18064–18069. 10.1073/pnas.1314928110. - DOI - PMC - PubMed
-
- Jannin S.; Bornet A.; Melzi R.; Bodenhausen G. High Field Dynamic Nuclear Polarization at 6.7T: Carbon-13 Polarization above 70% within 20min. Chem. Phys. Lett. 2012, 549, 99–102. 10.1016/j.cplett.2012.08.017. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

