The integrated structure of care: evidence for the efficacy of models of clinical governance in the prevention of fragility fractures after recent sentinel fracture after the age of 50 years
- PMID: 37603196
- PMCID: PMC10442313
- DOI: 10.1007/s11657-023-01316-9
The integrated structure of care: evidence for the efficacy of models of clinical governance in the prevention of fragility fractures after recent sentinel fracture after the age of 50 years
Abstract
Randomized clinical trials and observational studies on the implementation of clinical governance models, in patients who had experienced a fragility fracture, were examined. Literature was systematically reviewed and summarized by a panel of experts who formulated recommendations for the Italian guideline.
Purpose: After experiencing a fracture, several strategies may be adopted to reduce the risk of recurrent fragility fractures and associated morbidity and mortality. Clinical governance models, such as the fracture liaison service (FLS), have been introduced for the identification, treatment, and monitoring of patients with secondary fragility fractures. A systematic review was conducted to evaluate the association between multidisciplinary care systems and several outcomes in patients with a fragility fracture in the context of the development of the Italian Guidelines.
Methods: PubMed, Embase, and the Cochrane Library were investigated up to December 2020 to update the search of the Scottish Intercollegiate Guidelines Network. Randomized clinical trials (RCTs) and observational studies that analyzed clinical governance models in patients who had experienced a fragility fracture were eligible. Three authors independently extracted data and appraised the risk of bias in the included studies. The quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation methodology. Effect sizes were pooled in a meta-analysis using random-effects models. Primary outcomes were bone mineral density values, antiosteoporotic therapy initiation, adherence to antiosteoporotic medications, subsequent fracture, and mortality risk, while secondary outcomes were quality of life and physical performance.
Results: Fifteen RCTs and 62 observational studies, ranging from very low to low quality for bone mineral density values, antiosteoporotic initiation, adherence to antiosteoporotic medications, subsequent fracture, mortality, met our inclusion criteria. The implementation of clinical governance models compared to their pre-implementation or standard care/non-attenders significantly improved BMD testing rate, and increased the number of patients who initiated antiosteoporotic therapy and enhanced their adherence to the medications. Moreover, the treatment by clinical governance model respect to standard care/non-attenders significantly reduced the risk of subsequent fracture and mortality. The integrated structure of care enhanced the quality of life and physical function among patients with fragility fractures.
Conclusions: Based on our findings, clinicians should promote the management of patients experiencing a fragility fracture through structured and integrated models of care. The task force has formulated appropriate recommendations on the implementation of multidisciplinary care systems in patients with, or at risk of, fragility fractures.
Keywords: Fracture liaison service; Fragility fracture; Secondary prevention; Systematic review.
© 2023. The Author(s).
Conflict of interest statement
GA declares personal fees from Theramex, Amgen, BMS, Lilly, Fresenius Kabi and Galapagos. LC declares personal fees from UCB Pharma, Abiogen Pharma, Bruno Farmaceutici, Sandoz, Metagenics. DG has received honoraria as consultant for Eli-Lilly, Organon, MSD Italia. SG has received honoraria as consultant for UCB Pharma. SM has received honoraria as consultant for UCB, Eli-Lilly, Amgen. MLB has received (i) honoraria from Amgen, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB; (ii) grants and/or speaker: Abiogen, Alexion, Amgen, Bruno Farmaceutici, Echolight, Eli Lilly, Kyowa Kirin, SPA, Theramex, UCB Pharma; (iii) consultant: Alexion, Amolyt, Bruno Farmaceutici, Calcilytix, Kyowa Kirin, UCB Pharma. GC received research support from the European Community (EC), the Italian Agency of Drug (AIFA), and the Italian Ministry for University and Research (MIUR). He took part to a variety of projects that were funded by pharmaceutical companies (i.e., Novartis, GSK, Roche, AMGEN, and BMS). He also received honoraria as member of Advisory Board from Roche. No other potential conflicts of interest relevant to this article were disclosed. MR declares personal fees from Amgen, ABBvie, BMS, Eli Lilly, Galapagos, Menarini, Novartis, Pfizer, Sandoz, Theramex and UCB outside the submitted work. RM took part to a project funded by Abiogen Pharma. GI received honoraria as speaker by Eli-Lilly, Menarini, UCB Pharma. The other authors declare that they have no conflict of interest.
Figures
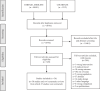





References
-
- Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393:364–376. - PubMed
-
- Wong RMY, Wong PY, Liu C, Wong HW, Chung YL, Chow SKH, et al. The imminent risk of a fracture-existing worldwide data: a systematic review and meta-analysis. Osteoporos Int. 2022;33(12):2453–2466. - PubMed
-
- Kanis JA, Cooper C, Rizzoli R, Reginster JY. Scientific Advisory Board of the European Society for Clinical and Economic Aspects of Osteoporosis (ESCEO) and the Committees of Scientific Advisors and National Societies of the International Osteoporosis Foundation (IOF). European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2019;30:3–44. - PMC - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

