Phase 3 Clinical Trial Comparing the Safety and Efficacy of Netarsudil to Ripasudil in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: Japan Rho Kinase Elevated Intraocular Pressure Treatment Trial (J-ROCKET)
- PMID: 37603205
- PMCID: PMC10499948
- DOI: 10.1007/s12325-023-02550-w
Phase 3 Clinical Trial Comparing the Safety and Efficacy of Netarsudil to Ripasudil in Patients with Primary Open-Angle Glaucoma or Ocular Hypertension: Japan Rho Kinase Elevated Intraocular Pressure Treatment Trial (J-ROCKET)
Abstract
Introduction: A clinical trial evaluated ocular hypotensive efficacy and safety of netarsudil 0.02% once daily (QD) relative to ripasudil 0.4% twice daily (BID).
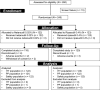
Methods: This was a single-masked, randomized, phase 3, superiority study. Japanese patients were randomized to either the netarsudil 0.02% group or the ripasudil 0.4% group in a 1:1 ratio and treated for 4 weeks. The primary efficacy variable was mean diurnal intraocular pressure (IOP) (average of diurnal time points at 09:00, 11:00, and 16:00) at Week 4.
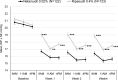
Results: A total of 245 patients were included in the primary analysis. At Week 4, least squares (LS) mean of diurnal IOP adjusted for baseline was 15.96 and 17.71 mmHg in the netarsudil 0.02% and ripasudil 0.4% groups, respectively, demonstrating the superiority of netarsudil 0.02% QD over ripasudil 0.4% BID by a margin of - 1.74 mmHg (p < 0.0001). Mean reduction from baseline in mean diurnal IOP at Week 4 was 4.65 and 2.98 mmHg, respectively. Adverse events (AEs) occurred less frequently in netarsudil 0.02% than in ripasudil 0.4%, with the incidence of ocular AEs being 59.8% and 66.7%, respectively. The most frequently reported AE was conjunctival hyperemia in both groups, with an incidence of 54.9% and 62.6%, respectively. No serious eye-related AEs were reported.
Conclusion: Netarsudil ophthalmic solution 0.02% dosed QD (p.m.) was well tolerated and more effective in reducing IOP than ripasudil ophthalmic solution 0.4% dosed BID. Netarsudil 0.02% QD may become an important option for the treatment of Japanese patients with primary open-angle glaucoma (POAG) or ocular hypertension (OHT).
Trial registration: ClinicalTrials.gov identifier, NCT04620135.
Keywords: Conjunctival hyperemia; Glaucoma; Intraocular pressure; Netarsudil; Rho-associated protein kinase; Ripasudil.
© 2023. The Author(s).
Conflict of interest statement
Makoto Araie is a consultant for Aerie and has received fees from Heidelberg Engineering, Alcon, Pfizer, Santen, Topcon Medical Systems, Otsuka, Senju, and Kowa, and holds patents/royalties with Topcon Medical Systems. Kazuhisa Sugiyama is a consultant for Aerie and has received lecture/manuscript fees from Otsuka, Santen, Senju, Viatris, Kowa, Novartis, Bayer, and Inami. Ryo Iwata, Casey Kopczynski, Michelle Senchyna are the employees of and stockholders in Aerie Pharmaceuticals (now Alcon). Kenji Aso (Sun Pharma Japan Ltd.), Koji Kanemoto (Alexion Pharma GK), and David A Hollander (Revance Therapeutics, Inc) were employees of Aerie Pharmaceuticals during the conduct of the study.
Figures



References
-
- Kass MA, Heuer DK, Higginbotham EJ, et al. The ocular hypertension treatment study: a randomized trial determines that topical ocular hypotensive medication delays or prevents the onset of primary open-angle glaucoma. Arch Ophthalmol. 2002;120:701–713. doi: 10.1001/archopht.120.6.701. - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

