Towards Model-Informed Precision Dosing of Voriconazole: Challenging Published Voriconazole Nonlinear Mixed-Effects Models with Real-World Clinical Data
- PMID: 37603216
- PMCID: PMC10520167
- DOI: 10.1007/s40262-023-01274-y
Towards Model-Informed Precision Dosing of Voriconazole: Challenging Published Voriconazole Nonlinear Mixed-Effects Models with Real-World Clinical Data
Abstract
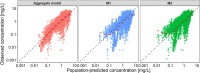
Background and objectives: Model-informed precision dosing (MIPD) frequently uses nonlinear mixed-effects (NLME) models to predict and optimize therapy outcomes based on patient characteristics and therapeutic drug monitoring data. MIPD is indicated for compounds with narrow therapeutic range and complex pharmacokinetics (PK), such as voriconazole, a broad-spectrum antifungal drug for prevention and treatment of invasive fungal infections. To provide guidance and recommendations for evidence-based application of MIPD for voriconazole, this work aimed to (i) externally evaluate and compare the predictive performance of a published so-called 'hybrid' model for MIPD (an aggregate model comprising features and prior information from six previously published NLME models) versus two 'standard' NLME models of voriconazole, and (ii) investigate strategies and illustrate the clinical impact of Bayesian forecasting for voriconazole.
Methods: A workflow for external evaluation and application of MIPD for voriconazole was implemented. Published voriconazole NLME models were externally evaluated using a comprehensive in-house clinical database comprising nine voriconazole studies and prediction-/simulation-based diagnostics. The NLME models were applied using different Bayesian forecasting strategies to assess the influence of prior observations on model predictivity.
Results: The overall best predictive performance was obtained using the aggregate model. However, all NLME models showed only modest predictive performance, suggesting that (i) important PK processes were not sufficiently implemented in the structural submodels, (ii) sources of interindividual variability were not entirely captured, and (iii) interoccasion variability was not adequately accounted for. Predictive performance substantially improved by including the most recent voriconazole observations in MIPD.
Conclusion: Our results highlight the potential clinical impact of MIPD for voriconazole and indicate the need for a comprehensive (pre-)clinical database as basis for model development and careful external model evaluation for compounds with complex PK before their successful use in MIPD.
© 2023. The Author(s).
Conflict of interest statement
Charlotte Kloft and Wilhelm Huisinga report grants from an industry consortium (AbbVie Deutschland GmbH & Co. K.G., AstraZeneca, Boehringer Ingelheim Pharma GmbH & Co. KG., Gruenenthal GmbH, F. Hoffmann-La Roche Ltd., Merck KGaA and Sanofi) for the graduate research training program PharMetrX. In addition, Charlotte Kloft reports research grants from the Innovative Medicines Initiative-Joint Undertaking (“DDMoRe”), from H2020-EU.3.1.3 (“FAIR”), Diurnal Ltd. and the Federal Ministry of Education and Research within the Joint Programming Initiative on Antimicrobial Resistance Initiative (“JPIAMR”), all outside the submitted work. Markus Zeitlinger received grants from Pfizer for other clinical studies, none of them associated with voriconazole. Franziska Kluwe is current employee of Boehringer Ingelheim Pharma GmbH & Co. KG. All other authors declare no competing interests for this work.
Figures





References
-
- World Health Organization (WHO). World Health Organization model list of essential medicines: 22nd list (2021) [Internet]. Geneva PP—Geneva: World Health Organization; 2021. https://apps.who.int/iris/handle/10665/345533.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

