Zinc Carnosine Metal-Organic Coordination Polymer as a Potent Broadly Active Influenza Vaccine Platform with In Vitro Shelf-Stability
- PMID: 37603310
- PMCID: PMC11654055
- DOI: 10.1021/acs.molpharmaceut.3c00424
Zinc Carnosine Metal-Organic Coordination Polymer as a Potent Broadly Active Influenza Vaccine Platform with In Vitro Shelf-Stability
Abstract
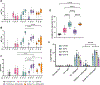
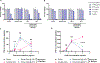
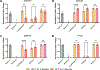
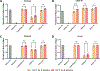
Current seasonal influenza vaccines are limited in that they need to be reformulated every year in order to account for the constant mutation of the virus. Hemagglutinin (HA) immunogens have been developed using a computationally optimized broadly reactive antigen (COBRA) methodology, which are able to elicit an antibody response that neutralizes antigenically distinct influenza strains; however, subunit proteins are not immunogenic enough on their own to generate a substantial immune response. Due to this, different delivery strategies and adjuvants can be used to improve immunogenicity. Recently, we reported a new coordination polymer composed of the dipeptide carnosine and zinc (ZnCar) that is able to deliver protein antigens along with CpG to generate a potent immune response. In the present work, ZnCar was used to deliver the COBRA HA immunogen Y2 and the adjuvant CpG. We incorporated Y2 into ZnCar using two different methods to assess which would be the most immunogenic. Mice vaccinated with Y2 and CpG complexed with ZnCar showed an improved humoral and cellular response when compared to mice vaccinated with soluble Y2 and CpG. Further, we demonstrate in vitro that when Y2 and CpG are coordinated with ZnCar, they are protected from degradation at 40 °C for 3 months or 24 °C for 6 months. Overall, ZnCar shows promise as a delivery vehicle for subunit vaccines, given its superior immunogenicity and in vitro storage stability.
Keywords: broadly active antigen; coordination polymer; influenza; storage stability; vaccine.
Conflict of interest statement
Conflict of Interest Statement
The authors have no conflict of interest to disclose.
Figures



Similar articles
-
Enhancement of subunit vaccine delivery with zinc-carnosine coordination polymer through the addition of mannan.Int J Pharm. 2024 May 10;656:124076. doi: 10.1016/j.ijpharm.2024.124076. Epub 2024 Apr 1. Int J Pharm. 2024. PMID: 38569976 Free PMC article.
-
Metal-Organic Coordination Polymer for Delivery of a Subunit Broadly Acting Influenza Vaccine.ACS Appl Mater Interfaces. 2022 Jun 29;14(25):28548-28558. doi: 10.1021/acsami.2c04671. Epub 2022 Jun 15. ACS Appl Mater Interfaces. 2022. PMID: 35704854 Free PMC article.
-
Sustained delivery of CpG oligodeoxynucleotide by acetalated dextran microparticles augments effector response to Computationally Optimized Broadly Reactive Antigen (COBRA) influenza hemagglutinin.Int J Pharm. 2023 Jan 5;630:122429. doi: 10.1016/j.ijpharm.2022.122429. Epub 2022 Nov 25. Int J Pharm. 2023. PMID: 36436743 Free PMC article.
-
Next-Generation Influenza HA Immunogens and Adjuvants in Pursuit of a Broadly Protective Vaccine.Viruses. 2021 Mar 24;13(4):546. doi: 10.3390/v13040546. Viruses. 2021. PMID: 33805245 Free PMC article. Review.
-
[Application and prospect of natural active ingredients of traditional Chinese medicine in immunological adjuvant for influenza vaccine].Zhongguo Zhong Yao Za Zhi. 2023 Nov;48(22):5985-5992. doi: 10.19540/j.cnki.cjcmm.20230823.602. Zhongguo Zhong Yao Za Zhi. 2023. PMID: 38114204 Review. Chinese.
Cited by
-
Clinical and Preclinical Methods of Heat-Stabilization of Human Vaccines.Mol Pharm. 2024 Mar 4;21(3):1015-1026. doi: 10.1021/acs.molpharmaceut.3c00844. Epub 2024 Jan 30. Mol Pharm. 2024. PMID: 38288698 Free PMC article. Review.
-
Enhancement of subunit vaccine delivery with zinc-carnosine coordination polymer through the addition of mannan.Int J Pharm. 2024 May 10;656:124076. doi: 10.1016/j.ijpharm.2024.124076. Epub 2024 Apr 1. Int J Pharm. 2024. PMID: 38569976 Free PMC article.
-
The potential of nano- and microparticle-based influenza vaccines with enhanced shelf lives.Nanomedicine (Lond). 2024;19(26):2135-2138. doi: 10.1080/17435889.2024.2392484. Epub 2024 Oct 8. Nanomedicine (Lond). 2024. PMID: 39377099 No abstract available.
-
Controlled Release of Poly(U) via Acetalated Dextran Microparticles for Enhanced Vaccine Adjuvant Delivery.bioRxiv [Preprint]. 2025 Jul 21:2025.07.16.665134. doi: 10.1101/2025.07.16.665134. bioRxiv. 2025. PMID: 40777285 Free PMC article. Preprint.
-
Optimizing delivery in a multivalent subunit influenza vaccine using mixed polymeric microparticle degradation rates.J Control Release. 2025 Aug 10;384:113936. doi: 10.1016/j.jconrel.2025.113936. Epub 2025 Jun 6. J Control Release. 2025. PMID: 40482923
References
-
- CDC Disease Burden of Flu. https://www.cdc.gov/flu/about/burden/index.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

