Immune-Related Diarrhea and Colitis in Non-small Cell Lung Cancers: Impact of Multidisciplinary Management in a Real-World Setting
- PMID: 37603442
- PMCID: PMC10769780
- DOI: 10.1093/oncolo/oyad238
Immune-Related Diarrhea and Colitis in Non-small Cell Lung Cancers: Impact of Multidisciplinary Management in a Real-World Setting
Abstract
Introduction: Immune-related adverse events (irAEs) constitute a challenge in the clinical management of solid tumors. This study aims to collect real-world data on the occurrence of immune-mediated diarrhea and colitis (IMDC) in advanced non-small cell lung cancer (aNSCLC) treated with immune checkpoint inhibitors (ICIs) and to assess the clinical impact of a multidisciplinary approach (MDA) on IMDC management.
Methods: We retrospectively collected data on patients with aNSCLC consecutively treated with ICIs, either as single agent or in combination with chemotherapy, between September 2013 and July 2022. Among patients developing IMDC, we conducted blinded revision of colonic biopsies and evaluated the clinical impact of the introduction of MDA through predefined indicators.
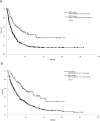
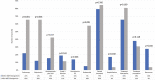
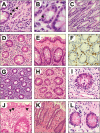
Results: Among the 607 patients included, 84 (13.8%) experienced IMDC. Pathological review highlighted a high prevalence of microscopic colitis (28%), with a collagenous pattern linked to longer symptoms duration (P = .01). IMDC occurred more frequently in females (P = .05) and PD-L1 expressors (P = .014) and was correlated with longer progression-free survival (17.0 vs 5.8, P < .001) and overall survival (28.3 vs 9.5, P < .001). The introduction of MDA was associated with increased employment of diagnostical tools such as fecal calprotectin test (P < .001), colonoscopy (P < .001), and gastroenterological evaluation (P = .017) and a significant decrease in both grade 3 conversion rate (P = .046) and recurrence after rechallenge (P = .016). Hospitalization rate dropped from 17.2% to 3.8% (P: ns).
Conclusion: These findings highlight the clinical relevance of IMDC and support the incorporation of a MDA to optimize the clinical management of this irAE to improve patient care. Prospective validation has been planned.
Keywords: collagenous colitis; immune checkpoint inhibitors; immune-mediated diarrhea and colitis; immune-related adverse events; immunotherapy rechallenge.
© The Author(s) 2023. Published by Oxford University Press.
Conflict of interest statement
Laura Bonanno declares speaker fee and participation to advisory board for AstraZeneca, Roche, MSD, Bristol-Myers Squibb, Sanofy, Novartis, outside the submitted work; is part of the steering committee for AstraZeneca; and received travel expenses from AstraZeneca and Roche. Davide Massa has received travel expenses from Eli Lilly. Giulia Pasello declares speaker fee and participation to advisory board for Amgen, AstraZeneca, BMS, Lilly, MSD, Novartis, Roche, and Janssen, and unconditioned research support from AstraZeneca and Roche. Matteo Fassan received grants from QED Therapeutics, grants and personal fees from Astellas Pharma, and personal fees from Tesaro—GSK, Diaceutics, and Roche. Edoardo Vincenzo Savarino declares speaker fees from Abbvie, Agave, AGPharma, Alfasigma, Aurora Pharma, CaDiGroup, Celltrion, Dr Falk, EG Stada Group, Fenix Pharma, Fresenius Kabi, Galapagos, Janssen, JB Pharmaceuticals, Innovamedica/Adacyte, Malesci, Mayoly Biohealth, Omega Pharma, Pfizer, Reckitt Benckiser, Sandoz, SILA, Sofar, Takeda, Tillots, Unifarco; has served as consultant for Abbvie, Agave, Alfasigma, Biogen, Bristol-Myers Squibb, Celltrion, Diadema Farmaceutici, Dr. Falk, Fenix Pharma, Fresenius Kabi, Janssen, JB Pharmaceuticals, Merck & Co, Nestlè, Reckitt Benckiser, Regeneron, Sanofi, SILA, Sofar, Synformulas GmbH, Takeda, Unifarco; and research support from Pfizer, Reckitt Benckiser, SILA, Sofar, Unifarco, and Zeta Farmaceutici. Valentina Guarneri declares speaker fees from Eli Lilly, Exact Sciences, Novartis, Pfizer, Gilead, MSD, Amgen, Sanofi, Merck Serono, and Eisai outside the submitted work. The other authors indicated no financial relationships.
Figures
Similar articles
-
Impact of antibiotic therapy on the development and response to treatment of immune checkpoint inhibitor-mediated diarrhea and colitis.J Immunother Cancer. 2019 Sep 5;7(1):242. doi: 10.1186/s40425-019-0714-x. J Immunother Cancer. 2019. PMID: 31488205 Free PMC article.
-
Incidence of immune checkpoint inhibitor-mediated diarrhea and colitis (imDC) in patients with cancer and preexisting inflammatory bowel disease: a propensity score-matched retrospective study.J Immunother Cancer. 2021 Jun;9(6):e002567. doi: 10.1136/jitc-2021-002567. J Immunother Cancer. 2021. PMID: 34158318 Free PMC article.
-
Outcomes of Immune Checkpoint Inhibitor-related Diarrhea or Colitis in Cancer Patients With Superimposed Gastrointestinal Infections.Am J Clin Oncol. 2021 Aug 1;44(8):402-408. doi: 10.1097/COC.0000000000000841. Am J Clin Oncol. 2021. PMID: 34107499
-
Immune Checkpoint Inhibitor-Mediated Diarrhea and Colitis: A Clinical Review.JCO Oncol Pract. 2020 Aug;16(8):453-461. doi: 10.1200/OP.20.00002. Epub 2020 Jun 25. JCO Oncol Pract. 2020. PMID: 32584703 Review.
-
The incidence and relative risk of PD-1/PD-L1 inhibitors-related colitis in non-small cell lung cancer: A meta-analysis of randomized controlled trials.Int Immunopharmacol. 2019 Dec;77:105975. doi: 10.1016/j.intimp.2019.105975. Epub 2019 Nov 6. Int Immunopharmacol. 2019. PMID: 31704288 Review.
Cited by
-
Endoscopic findings of immune checkpoint inhibitor-related gastrointestinal adverse events.Clin Endosc. 2024 Nov;57(6):725-734. doi: 10.5946/ce.2024.003. Epub 2024 Aug 29. Clin Endosc. 2024. PMID: 39206499 Free PMC article. Review.
-
A Multidisciplinary Approach in the Management of Infectious Diarrhea in the Emergency Department.Cureus. 2024 Aug 26;16(8):e67788. doi: 10.7759/cureus.67788. eCollection 2024 Aug. Cureus. 2024. PMID: 39323695 Free PMC article.
-
Immune-related adverse events requiring hospitalization in patients with lung cancer: implications and insights.Oncologist. 2024 Nov 4;29(11):e1615-e1620. doi: 10.1093/oncolo/oyae189. Oncologist. 2024. PMID: 39066589 Free PMC article.
-
Immune checkpoint inhibitor-induced anti-Hu antibody-associated gastrointestinal pseudo-obstruction: a case report and literature review.Front Immunol. 2025 Apr 1;16:1555790. doi: 10.3389/fimmu.2025.1555790. eCollection 2025. Front Immunol. 2025. PMID: 40236696 Free PMC article. Review.
-
CD8+ cell dominance in immune checkpoint inhibitor-induced colitis and its heterogeneity across endoscopic features.Therap Adv Gastroenterol. 2024 Dec 23;17:17562848241309445. doi: 10.1177/17562848241309445. eCollection 2024. Therap Adv Gastroenterol. 2024. PMID: 39735350 Free PMC article.
References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

