The small non-coding RNA B11 regulates multiple facets of Mycobacterium abscessus virulence
- PMID: 37603560
- PMCID: PMC10470900
- DOI: 10.1371/journal.ppat.1011575
The small non-coding RNA B11 regulates multiple facets of Mycobacterium abscessus virulence
Abstract
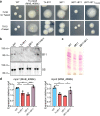
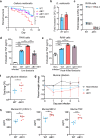
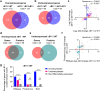
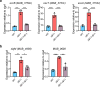
Mycobacterium abscessus causes severe disease in patients with cystic fibrosis. Little is known in M. abscessus about the roles of small regulatory RNAs (sRNA) in gene regulation. We show that the sRNA B11 controls gene expression and virulence-associated phenotypes in this pathogen. B11 deletion from the smooth strain ATCC_19977 produced a rough strain, increased pro-inflammatory signaling and virulence in multiple infection models, and increased resistance to antibiotics. Examination of clinical isolate cohorts identified isolates with B11 mutations or reduced expression. We used RNAseq and proteomics to investigate the effects of B11 on gene expression and test the impact of mutations found in clinical isolates. Over 200 genes were differentially expressed in the deletion mutant. Strains with the clinical B11 mutations showed expression trends similar to the deletion mutant, suggesting partial loss of function. Among genes upregulated in the B11 mutant, there was a strong enrichment for genes with B11-complementary sequences in their predicted ribosome binding sites (RBS), consistent with B11 functioning as a negative regulator that represses translation via base-pairing to RBSs. Comparing the proteomes similarly revealed that upregulated proteins were strongly enriched for B11-complementary sequences. Intriguingly, genes upregulated in the absence of B11 included components of the ESX-4 secretion system, critical for M. abscessus virulence. Many of these genes had B11-complementary sequences at their RBSs, which we show is sufficient to mediate repression by B11 through direct binding. Altogether, our data show that B11 acts as a direct negative regulator and mediates (likely indirect) positive regulation with pleiotropic effects on gene expression and clinically important phenotypes in M. abscessus. The presence of hypomorphic B11 mutations in clinical strains is consistent with the idea that lower B11 activity may be advantageous for M. abscessus in some clinical contexts. This is the first report on an sRNA role in M. abscessus.
Copyright: © 2023 Bar-Oz et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
-
- Maiz L., Giron R., Olveira C., Vendrell M., Nieto R. and Martinez-Garcia M.A. (2016) Prevalence and factors associated with nontuberculous mycobacteria in non-cystic fibrosis bronchiectasis: a multicenter observational study. BMC Infect Dis, 16, 437. doi: 10.1186/s12879-016-1774-x - DOI - PMC - PubMed
-
- Floto R.A., Olivier K.N., Saiman L., Daley C.L., Herrmann J.L., Nick J.A. et al. (2016) US Cystic Fibrosis Foundation and European Cystic Fibrosis Society consensus recommendations for the management of non-tuberculous mycobacteria in individuals with cystic fibrosis: executive summary. Thorax, 71, 88–90. doi: 10.1136/thoraxjnl-2015-207983 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

