NLRP3 is essential for neutrophil polarization and chemotaxis in response to leukotriene B4 gradient
- PMID: 37603754
- PMCID: PMC10468616
- DOI: 10.1073/pnas.2303814120
NLRP3 is essential for neutrophil polarization and chemotaxis in response to leukotriene B4 gradient
Abstract
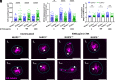
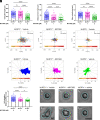
Neutrophil recruitment to sites of infection and inflammation is an essential process in the early innate immune response. Upon activation, a subset of neutrophils rapidly assembles the multiprotein complex known as the NLRP3 inflammasome. The NLRP3 inflammasome forms at the microtubule organizing center, which promotes the formation of interleukin (IL)-1β and IL-18, essential cytokines in the immune response. We recently showed that mice deficient in NLRP3 (NLRP3-/-) have reduced neutrophil recruitment to the peritoneum in a model of thioglycolate-induced peritonitis. Here, we tested the hypothesis that this diminished recruitment could be, in part, the result of defects in neutrophil chemotaxis. We find that NLRP3-/- neutrophils show loss of cell polarization, as well as reduced directionality and velocity of migration toward increasing concentrations of leukotriene B4 (LTB4) in a chemotaxis assay in vitro, which was confirmed through intravital microscopy of neutrophil migration toward a laser-induced burn injury of the liver. Furthermore, pharmacologically blocking NLRP3 inflammasome assembly with MCC950 in vitro reduced directionality but preserved nondirectional movement, indicating that inflammasome assembly is specifically required for polarization and directional chemotaxis, but not cell motility per se. In support of this, pharmacological breakdown of the microtubule cytoskeleton via nocodazole treatment induced cell polarization and restored nondirectional cell migration in NLRP3-deficient neutrophils in the LTB4 gradient. Therefore, NLRP3 inflammasome assembly is required for establishment of cell polarity to guide the directional chemotactic migration of neutrophils.
Keywords: NLRP3 inflammasome; cell polarization; chemotaxis; neutrophils.
Conflict of interest statement
The authors declare no competing interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

