Preferences of Patients and Providers in High-Burden Malaria Settings for Long-Acting Malaria Chemoprevention
- PMID: 37604474
- PMCID: PMC10551098
- DOI: 10.4269/ajtmh.23-0245
Preferences of Patients and Providers in High-Burden Malaria Settings for Long-Acting Malaria Chemoprevention
Abstract
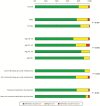
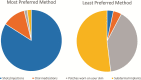
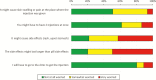
Antimalarial medications are recommended for chemoprevention as part of malaria control programs to decrease the morbidity and mortality related to more than 200 million infections each year. We sought to evaluate patient and provider acceptability of malaria chemoprevention in a long-acting formulation. We administered questionnaires to patients and providers in malaria endemic districts in Kenya and Zambia. Questions explored preferences and concerns around long-acting antimalarial formulations compared with oral formulations. We recruited 202 patient respondents (Kenya, n = 102; Zambia, n = 100) and 215 provider respondents (Kenya, n = 105; Zambia, n = 110). Long-acting injection was preferred to oral pills, whereas oral pills were preferred to implant or transdermal administration by patient respondents. Of 202 patient respondents, 80% indicated that they 'definitely would try' malaria chemoprevention offered by injection instead of oral pills. Of parents or guardians, 84% of 113 responded that they 'definitely would' have their child age < 12 years and 90% of 88 'definitely would' have their child ≥12 years receive an injection for malaria prevention. Provider respondents indicated that they would be more likely to prescribe a long-acting injectable product compared with an oral product for malaria chemoprevention in adults (70%), adolescents ages 12 years and older (67%), and children <12 years (81%). Potential for prolonged adverse effects with long-acting products was the highest concern for patient respondents, while higher medication-related cost was cited as the most concerning barrier to implementation by providers. Overall, these findings indicate enthusiasm for the development of long-acting injectable antimalarials to provide individual delivery method options across age groups.
Conflict of interest statement
Disclosures: A. O. and S. R. are directors of Tandem Nano Ltd and co-inventors of drug delivery patents including an atovaquone long-acting injectable formulation (PCT/GB2017/051746). S. R. has been co-investigator on funding received by the University of Liverpool from AstraZeneca, ViiV Healthcare, and Gilead Sciences. SR has received personal fees from Gilead Sciences. A. O. has been co-investigator on funding received by the University of Liverpool from ViiV Healthcare and Gilead Sciences. A. O. has received personal fees from Gilead and Assembly Biosciences. S. S. reports research funding to her institution from ViiV Healthcare. All other authors report no potential conflicts.
Figures



References
-
- World Health Organization , 2022. World Malaria Report. Geneva, Switzerland: WHO Global Malaria Programme.
-
- World Health Organization , 2021. Global Technical Strategy for Malaria 2016–2030, 2021 Update. Geneva, Switzerland: WHO.
-
- World Health Organization , 2019. High Burden to High Impact: A Targeted Malaria Response. Geneva, Switzerland: WHO.
-
- Phillips MA, Goldberg DE, 2018. Toward a chemical vaccine for malaria. Science 362: 1112–1113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

