Severe Hemolysis during Primaquine Radical Cure of Plasmodium vivax Malaria: Two Systematic Reviews and Individual Patient Data Descriptive Analyses
- PMID: 37604475
- PMCID: PMC10551063
- DOI: 10.4269/ajtmh.23-0280
Severe Hemolysis during Primaquine Radical Cure of Plasmodium vivax Malaria: Two Systematic Reviews and Individual Patient Data Descriptive Analyses
Abstract
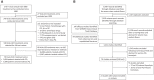
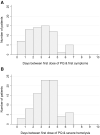
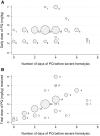
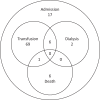
Primaquine (PQ) kills Plasmodium vivax hypnozoites but can cause severe hemolysis in patients with glucose-6-phosphate dehydrogenase (G6PD) deficiency. We conducted two systematic reviews. The first used data from clinical trials to determine the variety of definitions and frequency of hematological serious adverse events (SAEs) related to PQ treatment of vivax malaria. The second used data from prospective studies and case reports to describe the clinical presentation, management, and outcome of severe PQ-associated hemolysis necessitating hospitalization. In the first review, SAEs were reported in 70 of 249 clinical trials. There were 34 hematological SAEs among 9,824 patients with P. vivax malaria treated with PQ, nine of which necessitated hospitalization or blood transfusion. Criteria used to define SAEs were diverse. In the second review, 21 of 8,487 articles screened reported 163 patients hospitalized after PQ radical cure; 79.9% of whom (123 of 154) were prescribed PQ at ≥ 0.5 mg/kg/day. Overall, 101 patients were categorized as having probable or possible severe PQ-associated hemolysis, 96.8% of whom were G6PD deficient (< 30% activity). The first symptoms of hemolysis were reported primarily on day 2 or 3 (45.5%), and all patients were hospitalized within 7 days of PQ commencement. A total of 57.9% of patients (77 of 133) had blood transfusion. Seven patients (6.9%) with probable or possible hemolysis died. Even when G6PD testing is available, enhanced monitoring for hemolysis is warranted after PQ treatment. Clinical review within the first 5 days of treatment may facilitate early detection and management of hemolysis. More robust definitions of severe PQ-associated hemolysis are required.
Conflict of interest statement
The funders of the study had no role in study design, data collection, analysis, interpretation of data, or writing of the report.
Original studies received ethical approval via the relevant approval authorities. Ethical approval for this study was obtained from the Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research (HREC reference no. 2020-3935).
Figures
References
-
- World Health Organization , 2015. Guidelines for the Treatment of Malaria, 3rd edition. Geneva, Switzerland: WHO.
-
- Luzzatto L, Ally M, Notaro R, 2020. Glucose-6-phosphate dehydrogenase deficiency. Blood 136: 1225–1240. - PubMed
-
- Recht J, Ashley E, White NJ, 2014. Safety of 8-Aminoquinoline Antimalarial Medicines. Geneva, Switzerland: World Health Organization.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

