Randomized Trial Comparing Low- vs High-Dose IV Dexamethasone for Patients With Moderate to Severe Migraine
- PMID: 37604662
- PMCID: PMC10573135
- DOI: 10.1212/WNL.0000000000207648
Randomized Trial Comparing Low- vs High-Dose IV Dexamethasone for Patients With Moderate to Severe Migraine
Abstract
Background and objectives: Dexamethasone decreases the frequency of migraine recurrence after emergency department (ED) discharge. However, the optimal dose of dexamethasone is unknown. We hypothesized that dexamethasone 16 mg IV would allow greater rates of sustained headache relief than 4 mg when coadministered with metoclopramide 10 mg IV.
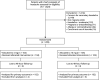
Methods: This was a randomized double-blind study. Adults who presented with a headache meeting International Classification of Headache Disorders, 3rd edition, migraine criteria were eligible if they rated the headache as moderate or severe in intensity. Pain intensity was assessed for up to 2 hours in the ED and through telephone 48 hours and 7 days later. The primary outcome was sustained headache relief. Secondary outcomes included headache relief within 2 hours and the number of headache days during the subsequent week. Relying on a priori criteria, the data safety monitoring committee recommended halting the study early for futility.
Results: A total of 1,823 patients were screened, and 209 patients were randomized. The mean age was 38 years (SD 11). One hundred seventy-nine of 209 (86%) identified as women. One hundred fifty-one of 209 (72%) of the population reported severe intensity; the rest reported moderate. Thirty-five of 102 (34%) participants in the metoclopramide +4 mg arm achieved sustained headache relief as did 42/102 (41%) participants in the metoclopramide +16 mg arm (absolute difference 7%, 95% CI -6% to 20%). Headache relief within 2 hours occurred in 77/104 (74%) low-dose and 82/105 (78%) high-dose participants (absolute difference 4%, 95% CI -8% to 16%). During the week after ED discharge, low-dose participants reported a median of 2 headache days (25th, 75th percentile 1, 5); in the high-dose arm, this was also 2 (25th, 75th percentile 0, 4) (mean difference 0.4, 95% CI -0.3 to 1.2).
Discussion: When added to 10 mg IV metoclopramide, doses of dexamethasone greater than 4 mg are unlikely to benefit patients in the ED with migraine.
Trial registration information: This study was registered at ClinicalTrials.gov on October 2, 2019 (NCT04112823). The first patient was enrolled on December 22, 2019.
Classification of evidence: This study provides Class I evidence that 16 mg of IV dexamethasone is unlikely to provide greater rates of sustained headache relief than 4 mg of IV dexamethasone among patients in the ED with migraine treated concurrently with IV metoclopramide.
© 2023 American Academy of Neurology.
Conflict of interest statement
The authors report no relevant disclosures. Go to
Figures
References
-
- Latev A, Friedman BW, Irizarry E, et al. . A randomized trial of a long-acting depot corticosteroid versus dexamethasone to prevent headache recurrence among patients with acute migraine who are discharged from an emergency department. Ann Emerg Med. 2019;73(2):141-149. doi:10.1016/j.annemergmed.2018.09.028 - DOI - PubMed
-
- Colman I, Friedman BW, Brown MD, et al. . Parenteral dexamethasone for acute severe migraine headache: meta-analysis of randomised controlled trials for preventing recurrence. BMJ. 2008;336(7657):1359-1361. doi:10.1136/bmj.39566.806725.be - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials