C3G and Ig-MPGN-treatment standard
- PMID: 37604793
- PMCID: PMC10828209
- DOI: 10.1093/ndt/gfad182
C3G and Ig-MPGN-treatment standard
Abstract
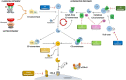
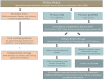
Among the broad spectrum of membranoproliferative glomerulonephritis (MPGN), immunofluorescence distinguishes C3 glomerulopathy (C3G), with predominant C3 deposits, and immunoglobulin-associated MPGN (Ig-MPGN), with combined C3 and Ig. However, there are several intersections between C3G and Ig-MPGN. Primary C3G and Ig-MPGN share the same prevalence of low serum C3 levels and of abnormalities of the alternative pathway of complement, and patients who present a bioptic pattern of Ig-MPGN at onset may show a C3G pattern in a subsequent biopsy. There is no specific therapy for primary C3G and Ig-MPGN and prognosis is unfavourable. The only recommended indications are inhibitors of the renin-angiotensin system, lipid-lowering agents and other renoprotective agents. The other drugs used currently, such as corticosteroids and mycophenolate mofetil, are often ineffective. The anti-C5 monoclonal antibody eculizumab has been tested in several patients, with mixed results. One reason for the uncertainty is the extremely variable clinical course, most likely reflecting a heterogeneous pathogenesis. An unsupervised clustering analysis that included histologic, biochemical, genetic and clinical data available at onset in patients with primary C3G and Ig-MPGN identified four clusters characterized by specific pathogenic mechanisms. This approach may facilitate accurate diagnosis and development of targeted therapies. Several trials are ongoing with drugs targeting different molecules of the complement cascade, however it is important to consider which component of the cascade may be the most appropriate for each patient. We review the current standards of treatment and discuss novel developments in the pathophysiology, diagnosis, outcome prediction and management of C3G and Ig-MPGN.
Keywords: C3 glomerulopathy; complement inhibitory drugs; complement system; membranoproliferative glomerulonephritis; unsupervised cluster analysis.
© The Author(s) 2023. Published by Oxford University Press on behalf of the ERA.
Conflict of interest statement
G.R. has consultancy agreements with Boehringer Inghelheim, Janssen Pharmaceuticals, Akebia Therapeutics, Alexion Pharmaceuticals, Alnylam, Inception Science Canada and BioCryst Pharmaceuticals. M.N. has received honoraria from Alexion Pharmaceuticals for giving lectures, and for participating in advisory boards, and she has received research grants from Omeros, Gemini, Novartis and BioCryst Pharmaceuticals. The funding sources had no role in writing the review or in the decision to submit the paper for publication.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

