Erythropoietin re-wires cognition-associated transcriptional networks
- PMID: 37604818
- PMCID: PMC10442354
- DOI: 10.1038/s41467-023-40332-8
Erythropoietin re-wires cognition-associated transcriptional networks
Abstract
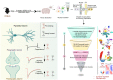
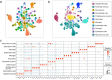
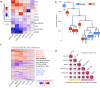
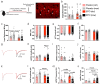
Recombinant human erythropoietin (rhEPO) has potent procognitive effects, likely hematopoiesis-independent, but underlying mechanisms and physiological role of brain-expressed EPO remained obscure. Here, we provide transcriptional hippocampal profiling of male mice treated with rhEPO. Based on ~108,000 single nuclei, we unmask multiple pyramidal lineages with their comprehensive molecular signatures. By temporal profiling and gene regulatory analysis, we build developmental trajectory of CA1 pyramidal neurons derived from multiple predecessor lineages and elucidate gene regulatory networks underlying their fate determination. With EPO as 'tool', we discover populations of newly differentiating pyramidal neurons, overpopulating to ~200% upon rhEPO with upregulation of genes crucial for neurodifferentiation, dendrite growth, synaptogenesis, memory formation, and cognition. Using a Cre-based approach to visually distinguish pre-existing from newly formed pyramidal neurons for patch-clamp recordings, we learn that rhEPO treatment differentially affects excitatory and inhibitory inputs. Our findings provide mechanistic insight into how EPO modulates neuronal functions and networks.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

