Simultaneous magnetic resonance imaging of pH, perfusion and renal filtration using hyperpolarized 13C-labelled Z-OMPD
- PMID: 37604826
- PMCID: PMC10442412
- DOI: 10.1038/s41467-023-40747-3
Simultaneous magnetic resonance imaging of pH, perfusion and renal filtration using hyperpolarized 13C-labelled Z-OMPD
Abstract
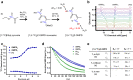
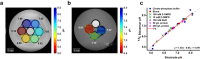
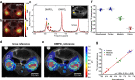
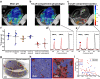
pH alterations are a hallmark of many pathologies including cancer and kidney disease. Here, we introduce [1,5-13C2]Z-OMPD as a hyperpolarized extracellular pH and perfusion sensor for MRI which allows to generate a multiparametric fingerprint of renal disease status and to detect local tumor acidification. Exceptional long T1 of two minutes at 1 T, high pH sensitivity of up to 1.9 ppm per pH unit and suitability of using the C1-label as internal frequency reference enables pH imaging in vivo of three pH compartments in healthy rat kidneys. Spectrally selective targeting of both 13C-resonances enables simultaneous imaging of perfusion and filtration in 3D and pH in 2D within one minute to quantify renal blood flow, glomerular filtration rates and renal pH in healthy and hydronephrotic kidneys with superior sensitivity compared to clinical routine methods. Imaging multiple biomarkers within a single session renders [1,5-13C2]Z-OMPD a promising new hyperpolarized agent for oncology and nephrology.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
SABRE-SHEATH Hyperpolarization of [1,5-13C2]Z-OMPD for Noninvasive pH Sensing.ACS Sens. 2024 Dec 27;9(12):6372-6381. doi: 10.1021/acssensors.4c01102. Epub 2024 Nov 18. ACS Sens. 2024. PMID: 39555976
-
Magnetic Resonance Imaging-Derived Renal Oxygenation and Perfusion During Continuous, Steady-State Angiotensin-II Infusion in Healthy Humans.J Am Heart Assoc. 2016 Mar 28;5(3):e003185. doi: 10.1161/JAHA.115.003185. J Am Heart Assoc. 2016. PMID: 27021686 Free PMC article.
-
Dual assessment of kidney perfusion and pH by exploiting a dynamic CEST-MRI approach in an acute kidney ischemia-reperfusion injury murine model.NMR Biomed. 2020 Jun;33(6):e4287. doi: 10.1002/nbm.4287. Epub 2020 Mar 9. NMR Biomed. 2020. PMID: 32153058
-
Functional Magnetic Resonance Imaging of the Kidneys-With and Without Gadolinium-Based Contrast.Adv Chronic Kidney Dis. 2017 May;24(3):162-168. doi: 10.1053/j.ackd.2017.03.006. Adv Chronic Kidney Dis. 2017. PMID: 28501079 Free PMC article. Review.
-
Measurement of single-kidney glomerular filtration function from magnetic resonance perfusion renography.Eur J Radiol. 2015 Aug;84(8):1419-1423. doi: 10.1016/j.ejrad.2015.05.009. Epub 2015 May 14. Eur J Radiol. 2015. PMID: 26032130 Review.
Cited by
-
Severely polarized extracellular acidity around tumour cells.Nat Biomed Eng. 2024 Jun;8(6):787-799. doi: 10.1038/s41551-024-01178-7. Epub 2024 Mar 4. Nat Biomed Eng. 2024. PMID: 38438799 Free PMC article.
-
3D virtual biopsy of in vivo pH and metabolism using PRESS and semi-LASER MRS of hyperpolarized 13C nuclei.Magn Reson Med. 2025 Sep;94(3):880-894. doi: 10.1002/mrm.30544. Epub 2025 May 1. Magn Reson Med. 2025. PMID: 40312850 Free PMC article.
-
Cascade-heterogated proton nanotransistors for multiplex pH-interval imaging.Nat Commun. 2025 Jul 1;16(1):5770. doi: 10.1038/s41467-025-61255-6. Nat Commun. 2025. PMID: 40593701 Free PMC article.
-
Detecting biomarkers by dynamic nuclear polarization enhanced magnetic resonance.Natl Sci Rev. 2024 Jun 29;11(9):nwae228. doi: 10.1093/nsr/nwae228. eCollection 2024 Sep. Natl Sci Rev. 2024. PMID: 39144741 Free PMC article. Review.
-
New Horizons in Hyperpolarized 13C MRI.Mol Imaging Biol. 2024 Apr;26(2):222-232. doi: 10.1007/s11307-023-01888-5. Epub 2023 Dec 26. Mol Imaging Biol. 2024. PMID: 38147265 Free PMC article.
References
-
- Lacava V, et al. Nephro-oncology: a link in evolution. Ren. Fail. 2015;37:1260–1266. - PubMed
-
- Rosner MH, Jhaveri KD, McMahon BA, Perazella MA. Onconephrology: The intersections between the kidney and cancer. CA Cancer J. Clin. 2021;71:47–77. - PubMed
-
- Huber V, et al. Cancer acidity: An ultimate frontier of tumor immune escape and a novel target of immunomodulation. Semin Cancer Biol. 2017;43:74–89. - PubMed
-
- McCarthy MF, Whitaker J. Manipulating Tumor Acidification as a Cancer Treatment Strategy. Alternative Med. Rev. 2010;15:264–272. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

