The non-classical major histocompatibility complex II protein SLA-DM is crucial for African swine fever virus replication
- PMID: 37604847
- PMCID: PMC10442341
- DOI: 10.1038/s41598-023-36788-9
The non-classical major histocompatibility complex II protein SLA-DM is crucial for African swine fever virus replication
Abstract
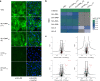
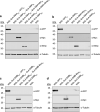
African swine fever virus (ASFV) is a lethal animal pathogen that enters its host cells through endocytosis. So far, host factors specifically required for ASFV replication have been barely identified. In this study a genome-wide CRISPR/Cas9 knockout screen in porcine cells indicated that the genes RFXANK, RFXAP, SLA-DMA, SLA-DMB, and CIITA are important for productive ASFV infection. The proteins encoded by these genes belong to the major histocompatibility complex II (MHC II), or swine leucocyte antigen complex II (SLA II). RFXAP and CIITA are MHC II-specific transcription factors, whereas SLA-DMA/B are subunits of the non-classical MHC II molecule SLA-DM. Targeted knockout of either of these genes led to severe replication defects of different ASFV isolates, reflected by substantially reduced plating efficiency, cell-to-cell spread, progeny virus titers and viral DNA replication. Transgene-based reconstitution of SLA-DMA/B fully restored the replication capacity demonstrating that SLA-DM, which resides in late endosomes, plays a crucial role during early steps of ASFV infection.
© 2023. The Author(s).
Conflict of interest statement
K.P., W.F., F.G., and J.K.B., are inventors on a pending patent application related to this work. The other authors declare that they have no competing interests.
Figures

References
-
- Montgomery R. On a form of swine fever occurring in British East Africa (Kenya Colony) J. Comp. Pathol. Ther. 1921;34:159–191. doi: 10.1016/s0368-1742(21)80031-4. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

